| Kingdom |
Fungi |
| Phylum |
Zygomycota |
| Class |
Zygomycetes |
| Order |
Mucorales |
| Family |
Mucoraceae |
Australia, Brazil, China, Colombia, Egypt, India, Italy, Japan, Kenya, Korea
Republic, Pakistan, Peru, Romania, USA, USSR and Yugoslavia.
Soft rot caused by R. stolonifer is a postharvest disease that can be
economically important without proper harvest and postharvest practices. It can
destroy storage roots that are bruised or wounded during harvest, left overnight
in the field after harvest, and are not properly cured before storage.
A water-soaked lesion starts to
develop a few hours after the root has been wounded or bruised during harvest or
handling. The lesion becomes covered by a web-like outgrowth with scattered
pinhead-like structures which are the mycelia
and sporangia of the fungus. In 4 to 5
days, the entire inner tissues of the root rot become soft, slimy and watery as
they liquefy. However, the periderm
remains almost intact except for small cracks and wrinkled appearance. Black
brush- like domes, which are the sporangiophores,
sporangia, and spores, emerge from these cracks, giving a whisker-like
appearance.
Rotting starts at either end where the fleshy root has been separated from
the rest of the stem and the root system during harvest but any bruise or a
slight scratch can initiate rotting.
Soft rot caused by Rhizopus has some similarities with bacterial soft
rot, as both produce the watery, flesh decaying rot and both attract fruit
flies. However, bacterial rot produces an unpleasant smell while Rhizopus rot
emits a pleasant fermented odour that attracts fruit flies. Also, bacterial rot
does not produce whiskers unlike Rhizopus soft rot
The pathogen first appear as white cottony colonies on potato-dextrose-agar
at 25°C becoming heavily speckled by the presence of sporangia
and then brownish black in age, spreading rapidly by means of stolons fixed at
various points to the substrate by rhizoids. The sporangiophores
measure up to 34 µm in diameter and 1000-3500 µm in length, are
smooth walled, non- septate, light brown, simple, and are in groups of 3-5 from
stolons opposite rhizoids. The sporangia are 100-350 µm in diameter,
globose or sub-globose with somewhat flattened base, white at first, then black
and have many spores. The columella measure
63-224 x 70-140 µm, are subglobose to dorsiventrally flattened, light
brownish grey, and are umbrella-shaped when dehisced. The collar are either
poorly defined or absent. The apophysis is present and visible below young
columellae. The rhizoids and stolons are transparent to dark brown. The
brownish-black sporangiospores measure (5) 8-10 (26) µm, are irregular
round, oval, elongate or angular in shape. They are heterotallic and strongly
striate with homogeneous content. The zygospores are produced when compatible
isolates are grown together. They measure 103-180 (220) µm, are globose,
or compressed between suspensors, brownish-black, thick walled and
verrucose. The suspensors measure 62-118 µm wide, swollen, and usually
unequal and somewhat granular. No growth is produced at 37°C (J.A.Lunn, 1974).
Rhizopus spp. is known to be omnipresent in the air as a contaminant.
Usually saprophytic, however, the
infection of fleshy sweetpotato roots occurs in the field through wounds or
bruises (made by harvesting implements) where the tissue is in a pre-necrotic
process.
Once a spore lands on a wounded tissue, it germinates and starts growing on
the surface, producing a thick mycelium which at the same time produces cell
degrading enzymes (pectinases, amylases) that denature the tissues in advance
thus leading to infection.
The infection process takes place in a wide temperature range of 20 to 30°C.
Moisture is critical and there should be enough moisture (75 to 85%) for spores
to germinate and mycelia to grow. Spores usually do not germinate at a higher
relative humidity . The optimum temperature for sporulation, mycelial growth,
and spore germination is between 23 to 28°C while rotting and tuber decay
develop between 15 and 23°C.
Exposure to sunlight and chilling can also predispose the storage roots
to the disease. A long exposure of fleshy roots to sunlight produces burns where
the development of infecting mycelia can occur. Chilling has a similar effect as
it damages the cell walls, through which the fungus can penetrate.
Since the disease does not affect the sweetpotato plant or roots while in the
field, the influence of environmental factors during the growing season is not
significant. However, conditions at harvest are important. For example, heavy
rain and low temperature at harvest make the possibility of infection higher.
Other species found in soft rot of sweetpotatoes are Rhizopus oryzae,
Mucor racemosus, M. circinelloides, and M. piriformis. All these
fungi are saprophytic so they initially produce pectic enzymes that break down
sweetpotato tissues.
Primary hosts are Cucurbitaceae and Solanum melongena (eggplant/aubergine).
It also attacks Abelmoschus esculentus (okra), Anona muricata
(soursop), Arachis hipogaea (groundnut), Arthrocarpus heterophyllus
(jackfruit), Cajanus cajan (pigeon pea), Carica papaya (papaw), Cicer
arietinum (chickpea), Cucumis melo (melon), Datura metel (Hindu
datura), Dacryodes edulis (Canarium saphii), Ficus carica (fig), Fragaria
spp. (strawberry), Gossypium spp. (cotton), Ipomoea batatas
(sweetpotato), Lufa aegiptica (loofah), Lycopersicon esculentum
(tomato), Malus domestica (apple),Oxalis tuberosa (oca),Pastinaca
sativa (parsnip), Prunus armeniaca (apricot), Prunus avium
(cherry), Prunus dulcis (bitter almond), Prunus domestica (plum), Prunus
persica (peach), Phyllantus emblica (Indian gooseberry), Pyrus
communis (European pear), sorghum, Tropaeolum tuberosum (mashua), Ullucus
tuberosus (ulluco), Vitis vinifera (grapes), Vicia faba (broad
bean), Vigna radiata (mungbean), Zea mays (maize) and Zingiber
officinale (ginger).
The fungus usually infects storage roots after harvest and a good diagnostic
sign is the presence of whiskers.
In the laboratory Rhizopus can be detected by placing a small piece of
wounded tissue in a moist chamber, and observing a mycelial mat outgrowth after
one to two days.
Rhizopus grows in any substrate containing starch and/or any kind of
sugar but grows better on PDA (Potato-Dextrose-Agar) plates.
Cultural control
Prevent unnecessary wounding during harvest and handling, and sorting out
wounded storage roots.
Not leaving
sweetpotatoes in the field after harvest. This will prevent unnecessary
exposure to direct sunlight, chilling in the field and other unfavourable
conditions.
Avoid harvesting
during or after rainfall.
Cure storage roots
immediately after harvest and storing them at 12 to 15°C. This counts only for
places where the fleshy roots are stored.
Host-Plant
Resistance
Some resistance has been reported but it may be compromised by excessive
wounding while susceptibility in various degrees has been observed.
Chemical control
2,6-dichloro-4-nitroaniline (DCNA) is used routinely as protective fungicide
for dipping roots to protect against rotting caused by Rhizopus.
Bouwkamp, J.C., Scott, L.E., and Kantzes, J.G. 1971. Control of soft rot (Rhizopus
spp.) in cut root pieces of sweetpotatoes with 2,6-dichloro-nitroaniline. Plant
Dis. Rep. 55:1097-1099.
Clark, C.A. and Moyer, J.W. 1988. Compendium of sweet potato diseases. APS
Press. 74 p.
Gatumbi, R.W. 1990. sweetpotato diseases in Kenya. Proceedings of a National
workshop held in Mombasa, Kenya, 7-ll May, 1990: 41-43.
Lunn, J.A. 1977. Rhizopus stolonifer. CMI Descriptions of Pathogenic
Fungi and Bacteria No. 524.
McClure, T.T. 1959. Rhizopus decay of sweetpotatoes as affected by chilling,
recuring and hydrowarming after storage. Phytopathology 49:359-361.
Palomar, M.K,
Solis, A.D., and Bandala, H.S.1980. Sweet potato tuber rot disease in the
Philippines. Ann. Trop. Res. 2:111-121.
Contributed by: Teresa
Ames
|
Taxonomy
Economic
importance
Geographical
distribution
Symptoms
Morphology
Biology
and ecology
Host
range
Detection
Management
References
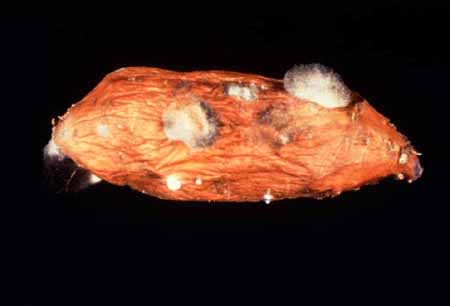
Web-like
outgrowth on storage root (W. Martin, APS). |

