|
Kingdom |
Virus |
|
Family |
Potyviridae |
|
Genus |
Potyvirus |
Although rarely recognised, SPFMV is responsible for considerable yield
reduction in sweetpotato crops worldwide. In the field, virus-free sweetpotato plants yield
from 20% to over 100% more than
infected plants. Sensitive cultivars may suffer significant yield losses
in comparison with tolerant ones.
Some isolates of SPFMV cause economic losses by their effect on storage root
quality (internal cork and russet crack).
Worldwide. SPFMV is found wherever sweetpotato is grown.
Symptoms of SPFMV on the foliage are generally slight or absent. The classic irregular chlorotic patterns (feathering) along
leaf veins
and faint-to-distinct chlorotic spots with or without purple margins occur in
some cultivars. Symptom visibility on foliage is influenced by cultivar
susceptibility, degree of stress, growth stage, and strain virulence.
Increased stress can lead to symptom expression, whereas rapid growth may result
in symptom remission. Symptoms on storage roots depend on the strain of SPFMV
and the sweetpotato variety. The common strain causes no symptom on storage
roots of any variety, but the “russet crack” and “internal cork” strains
cause external and internal dark necrotic lesions on certain varieties,
respectively.
The virion, ranging from 810 to 865 nm long, contains a single,
positive-sense strand of RNA with a Mr of approximately 3.7 x 106
D and a polypeptide with a of 36 to38 KD. It has shown a serological
relationship to some other potyviruses.
SPFMV is the most thoroughly characterized sweetpotato virus. SPFMV has many
of the biological and biochemical properties, and cytopathic characteristics of potyviruses,
including aphid transmissibility, occurrence of pinwheel inclusions, and a
relatively narrow host range. SPFMV is sap-transmissible, and transmitted by a
large number of aphid species such as Aphis gossypii, A. craccivora, Myzus
persicae, in a nonpersistent manner. Vegetative propagation perpetuates the
virus.
Several isolates and strains of SPFMV have been characterized in different
parts of the world; perhaps the most important ones being the ordinary (O),
russet crack (RC) and severe (S) strains because they directly affect storage
root quality.
SPFMV is found with SPCSV in several countries; the combination usually
results in a severe disease known as sweetpotato virus disease (SPVD).
Sweetpotato (Ipomoea batatas) is the main natural host of SPFMV,
although the virus occurs in wild Ipomoea species. The experimental host
range of the virus is mainly restricted to the Convolvulaceae and Chenopodiaceae,
but a few strains also infect species of the Solanaceae of which Nicotiana
benthamiana is a good propagation host for purification of the virus.
Several strains cause local lesions on Chenopodium amaranticolor and C.
quinoa.
Chenopodiaceae: Chenopodium murale, C. amaranticolor, C. quinoa, and
Spinacia oleracea (several strains).
Convolvulaceae: Calonyction aculeatun, Ipomoea hederaceae, I. incarnata,
I. lacunosa, I. purpurea, I. trichocarpa, I. tricolor, I. wrightii, Merremia
sibirica, and Quamoclit lobata.
Solanaceae: Datura
metel, Nicotiana benthamiana, N. clevelandii, N. occidentalis, and N.
tabacum (some strains).
Aphid control is not economically feasible. The main control measures are
avoidance of diseased plants as planting material, sanitation, and the use of
resistant varieties. As SPFMV is perpetuated between cropping cycles in infected
cuttings, the lack of symptoms in the foliage makes it difficult for farmers to
select SPFMV-free cuttings.
Ames, T.,
Smit, N.E.J.M., Braun, A.R., O’Sullivan, J.N., and Skoglund, L.G. 1996.
Sweetpotato: Major pests diseases, and nutritional disorders. International
Potato Center (CIP). Lima, Perú. 152 p.
Brunt, A.,Crabtree, K., and Gibbs, A. (eds.). 1990. Viruses of tropical
plants: Descriptions and lists from the VIDE database. CAB International,
Wallingford, UK.
Brunt, A.A.,
Crabtree, K., Dallwitz, M.J., Gibbs, A.J., Watson, L. and Zurcher, E.J. (eds.)
(1996 onwards). `Plant Viruses Online: Descriptions and Lists from the VIDE
Database. Version: 20th August 1996.' URL
http://biology.anu.edu.au/Groups/MES/vide/
Campbel, R.N.
Hall, D.H., and Mielines, N.M. 1974. Etiology of sweetpotato russet crack
disease, Phytopathology, 64:210-218.
Clark, C.A.,
Derrick, K.S., Pace, C.S., and Watson, B. 1986. Survey of wild Ipomoea
spp. as potential reservoirs of sweetpotato feathery mottle virus in Louisiana.
Plant Disease, 70: 931-932.
Clark, C.A., and
Moyer, J.M. 1988. Compendium of sweetpotato diseases. The American
Phytopahological Society. APS Press, Minnesota, USA. 74 p.
Moyer, J.W., and Cali, B.B. 1985. Properties of sweetpotato feathery mottle
virus RNA and capsid protein. Journal of General Virology, 66: 1185-1189.
Moyer, J.W., Cali, B.B., Kennedy, G.G., and Abou-Ghadir, F. 1980.
Identification of two sweetpotato feathery mottle virus strains in North
Carolina. Plant Disease, 64: 762-764.
Palomar, M.K., Barsalote, E.A. and Colis, H.S.V.
2000. Distribution, transmission and diseases of sweetpotato feathery
mottle virus. Ann. Trop. Res. 22 (1&2):16-30.
Usugi, T., Nakano, M., Onuki, M., Maoka, T., and Hayashi, T. 1994. A new
strain of sweetpotato feathery mottle virus that cause russet crack on fleshy
roots of some Japanese cultivars of sweetpotato. Ann. Phytopathol. Soc. Japan
60: 545-554.
Contributed
by: Segundo
Fuentes and Luis Salazar |
Taxonomy
Economic
importance
Geographical
distribution
Symptoms
Morphology
Ecology
Host
range
Management
References

Plant
with SPFMV showing yellowing of leaf veins ("vein clearing") (S. Fuentes
& L. Salazar).

Chlorotic spots on leaves with and without purple rings
(S.
Fuentes & L. Salazar).


Leaf vein feathering without pigmentation (Left - C. Clark) and bordered
with purple antocyanin pigment (Right - S.
Fuentes & L. Salazar).


Purpling around chlorotic
spots may be separated ring-spots (Left - E. van de Fliert) or fill the
area between spots (Right - R. Thijssen).


External root lesions induced by the
russet crack strain of SPFMV in storage roots (S. Fuentes & L. Salazar;
C. Clark).

Detail of russet crack lesions (E. Coleman)
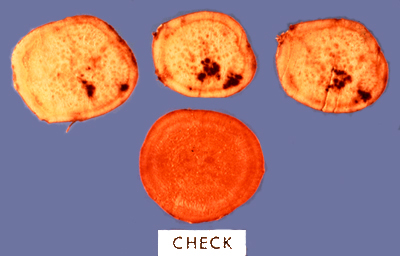
Internal root necrosis ("Internal
Cork") attributed to SPFMV (J. Moyer, APS). |

