|
Copper (Cu) deficiency in sweetpotato is encountered on some acid, sandy
soils of low total Cu content. It may also occur on calcareous
soils, in which Cu availability is low due to its insolubility at high pH.
In some organic soils, Cu may be tightly bound to soil constituents and poorly
available to plants. Liming of soils low in Cu can lead to the appearance of Cu
deficiency.
A number of visible symptoms of Cu deficiency have been observed on both
mature and young leaves, their extent and order of development varying among
cultivars. Conspicuous symptoms on the vines are usually associated with
considerable growth reduction. Chlorosis,
wilting and drooping of mature leaves may be the first visible symptom of Cu
deficiency. Leaves of intermediate age are first affected, but in time the
turnover of leaves will mean that the oldest leaves show symptoms. The chlorosis
is interveinal, with a gradual fading
of colour with distance from the main veins. Usually the minor veins retain less
green colour than the main veins, but may be sufficiently well defined to give
the chlorosis a mottled appearance. Chlorotic leaves may develop spots
or patches of necrosis, which spread
until the entire leaf is dead.
In some cultivars, necrotic spots have been observed on mature leaves without
prior chlorosis. Initially the necrotic spots are small, dark and sharply
defined. They may be clustered close to the point of petiole attachment, or more
commonly scattered over the leaf surface. The necrotic spots are clearly visible
on the lower side of the leaf, and are often adjacent to minor veins.
Subsequently, a yellow chlorosis develops around them, spreading to encompass a
number of lesions. Later, the area between the initial lesions becomes necrotic,
and eventually the whole leaf dies.
Symptoms affecting the young leaves and growing point may develop earlier or
later than those described above, and in some cultivars they may be the only
visible symptoms. Initially, the surface of young leaves may take on a silvery
appearance. A variety of leaf deformities may occur. New leaves are usually
small and may be misshapen, puckered, or thickened. They may develop holes due
to uneven expansion of the blade. Some reduction in internode length is common.
Cu-deficient sweetpotato plants may produce storage roots which are normal in
external appearance but contained brown streaks in the flesh (Pillai, et al.,
1986). Similar symptoms have also been observed in Cu-deficient crops in
northern Australia. Necrotic patches of vascular tissue may appear on the
surface as a brown area. Other roots, which appear healthy at harvest, may
develop subsurface necrosis followed by extensive tissue breakdown after a short
period of storage. Roots which appear sound may blacken rapidly after cutting.
These storage disorders may occur in crops showing little or no visible symptoms
on the vines.
Interveinal chlorosis on mature leaves, resulting from Cu deficiency, may
resemble deficiencies of Mg or Mn. In the case of Mg deficiency, the interveinal
pattern of chlorosis is usually more pronounced, and internodes near the tip may
be lengthened, whereas those in Cu-deficient plants are likely to be shortened.
In the case of Mn deficiency, the symptoms are more uniform throughout the plant
and do not generally progress to yellowing and senescence of mature leaves.
Zinc deficiency also causes the development of small, abnormally-shaped young
leaves, but Zn-deficient leaves are usually chlorotic, elongated, with reduced
lateral lobing. They are usually not asymmetrical or puckered, as is often the
case with Cu deficiency.
The critical concentration for Cu deficiency was found to be in the range 4-5
mg Cu/kg in the 7th to 9th youngest leaf blades. Concentrations below 2 mg Cu/kg
have been recorded in crops showing symptoms of Cu deficiency in northern
Australia.
Soil extraction using EDTA with ammonium bicarbonate has been recommended for
evaluation of Cu status, particularly in alkaline,
calcareous soils. Using this test, a
critical concentration of 0.3-0.4 mg Cu/kg was determined for wheat. Soil tests
have not been calibrated for Cu deficiency in sweetpotato.
A quick and inexpensive diagnostic test may be made by painting the surface
of a young leaf with a dilute solution containing Cu (eg. 0.25% CuSO4.5H2O +
0.25% Ca(OH)2). If Cu deficiency is the cause of the observed symptoms, the
treated leaf will show reduced symptoms and increased expansion of the lamina
over the following week. It is important to clearly label the leaf so that it
can be identified on later inspection. The result is most obviously seen if only
one half of the leaf is painted, so it can be compared with the untreated half.
A small amount of agricultural wetting agent or mild detergent may assist to
ensure even wetting of the leaf surface, but this is not essential with
sweetpotato, as the foliage wets relatively easily.
Cultural
control
Soil application or as foliar spray. On alkaline or organic soils,
foliar sprays may be more effective due to the rapid fixing of soil-applied Cu,
but in general, soil application is considered preferable. Rates of application
for sweetpotato crops have not been optimised. For other crops, soil
applications of copper sulfate at 1 kg Cu/ha on acid, sandy soils with low
organic matter content, to 7 kg Cu/ha on alkaline, peaty or heavy-textured soils
have been used. Overfertilisation with Cu can lead to Cu toxicity, so the
minimum effective dose should be sought. A single application of Cu may be
effective for up to 10 years.
For foliar application, rates as low as 0.25 kg Cu/ha have been sufficient
for wheat. Spray mixtures of 0.5% copper sulfate plus 0.5% hydrated lime or 0.5%
copper oxychloride have been recommended for ginger and vegetable crops,
respectively. Copper sulfate alone may cause leaf burn.
Asher, C.J. and Lee,
M.T. 1975. Nutritional disorders in ginger. Department of Agriculture,
University of Queensland, St. Lucia.
O’Sullivan, J., Loader, L., Asher,
C., Blamey, P. 1997b. Troubleshooting nutritional problems in a new industry:
sweet potato in North Queensland. Proceedings of the First Australian New Crops
Conference, Gatton, July 1996. Rural Industries Research and Development
Corporation, Australia.
O’Sullivan,
J.N., Asher, C.J. and Blamey, F.P.C. 1997. Nutrient Disorders of Sweet Potato.
ACIAR Monograph No. 48, Australian Centre for International Agricultural
Research, Canberra, 136 p.
Pillai, N.G., Mohankumar, B.,
Kabeerathumma, S. and Nair, P.G. 1986. Deficiency symptoms of micronutrients in
sweet potato (Ipomoea batatas L.). Journal of Root Crops 12 (2), 91-95.
Reuther, W. and Labanauskas, C.L.
1966. Copper. In: Chapman, H.D. (ed.) Diagnostic criteria for plants and soils.
Dept of Soils and Plant Nutrition, University of California Citrus Research
Centre and Agricultural Experiment Station, Riverside, California. pp 157-179.
Weir,
RG. and Cresswell, G.C. 1993. Plant Nutrient Disorders 3. Vegetable Crops. NSW
Agriculture; Inkata Press, Australia.
Contributed by:
Jane
O'Sullivan
|
Characteristics
and occurrence
Symptoms
Confusion
with other symptoms
Diagnostic
tests
Management
References

Mottled or patchy yellowing, necrotic spots and droopiness of mature
leaves.

Deformities, puckering and holes in young leaves.

Holes formed by the expansion of deformed tissue (J. O'Sullivan).

Newly opened leaves sometimes appear silvery (J. O'Sullivan).


Necrotic spots and chlorotic patches on mature leaves progress to
spreading necrosis (J. O'Sullivan).
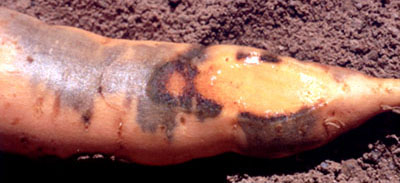
Patchy blackening of storage root cortical tissue just under the skin (J.
O'Sullivan).

Blackened sections of cortical tissue in roots after a period of storage
(L. Loader). |

