|
|
Class |
Secernentea |
|
Order |
Tylenchida |
|
Superfamily |
Tylenchoidea |
|
Family |
Meloidogynidae |
|
Genus |
Meloidogyne |
The degree of damage depends upon the population density of
the nematode, taxa present, susceptibility of the crop, and environmental
conditions, such as fertility, moisture and presence of other pathogenic
organisms, which may interact with nematodes. In sweetpotato an estimated annual
yield loss of 10.2 % was reported. In susceptible varieties pathogenicity
of Meloidogyne incognita showed a 50% storage root reduction at
a population density of 20,000/cm3. Aside from yield loss, cracking can make
storage roots unmarketable.
The root-knot nematode,
Meloidogyne incognita, is worldwide in distribution. It is widespread in
Asia, Southeast Asia and usually occurs in warmer areas. In some
countries, M. javanica is more dominant.
Above-ground symptoms exhibited by sweetpotato plants due to
root-knot nematode include poor shoot growth, leaf chlorosis and stunting.
Galling of rootlets and severe cracking of storage roots on some varieties or
formation of small bumps or blisters on other varieties are important
below-ground symptoms in sweetpotato. There may also be brown to black spots in the
outer layers of flesh which are not evident unless the storage root is peeled.
Presence can be diagnosed by the pearl-like swollen female
nematodes in flesh of storage roots or in fibrous roots, within the galls or
dark spots.
M. incognita is sexually dimorphic.
The female is saccate to globose,
0.4-1.3 mm. long, and usually embedded in root tissues which are often swollen
or galled. Its body is soft, pearl white in colour and does not form a
cyst. The neck protrudes anteriorly and the excretory pore is anterior to the
median bulb and often near the stylet base. Its vulva and anus are terminal,
flush with or slightly raised from the body contour, the cuticle of the terminal
region forms a characteristic perineal pattern, which is made up of the stunted
tail terminus, phasmids, lateral lines, vulva and anus surrounded by cuticular
striae; the pattern is often characteristic for individual species. The female
stylet is shorter, 10-24 Ám usually 14-15Ám, and more delicate with small
basal knobs. The paired gonads have extensive convoluted ovaries that fill most
of the swollen body cavity. There are six large unicellular rectal glands in the
posterior body which produce a gelatinous matrix, which is excreted via the
rectum to form an egg sac in which many eggs are deposited.
The male has long, thin, cylindrical shape of a worm but the
lip region has a distinct head cap, which includes a labial disc surrounded by
lateral and medial lips. The head skeleton is usually weaker and the stylet less
robust and shorter, 18-24 Ám long for many species. Infective second stage
juveniles, often free in the soil, are usually 0.3-0.5 mm long; they are less
robust, the stylet is delicate with small basal knobs, under 20 Ám long, and
the head skeleton weak. The median oesophageal bulb is well developed and the
oesophageal glands are extensive, overlapping the intestine for several body
widths, mainly ventrally; the tail is conoid, often ending in a narrow rounded
terminus, but tail length is variable, 1.5-7 anal body widths between species,
it often ends in a clear hyaline region, the extent of which can help to
distinguish species. (Sasser and Carter, 1985).
In addition to the adult and egg, there are four juvenile
stages and four moults in the life cycle of M. incognita. The first stage
juvenile develops in the egg, and the first moult usually occurs within the
eggshell, giving rise to the second-stage juvenile, which emerges free into the
soil or plant tissue. Once the nematode begins feeding on tissue of a favourable
host, the second, third and fourth moults occur giving rise to the third, fourth
and fifth or adult stages, respectively. Between moults, there is further growth
and development of the nematode, with concurrent development of the reproductive
systems in the two sexes. Upon maturity, the female deposits eggs and the life
cycle is repeated. Its life cycle is similar to Heterodera but the
generation time, 4-8 weeks, is shorter.
M. incognita has a very wide host range including weeds. Meloidogyne
spp. attack virtually all plants.
Cultural control
Crop rotation.
Non-host crops or resistant crops can be planted when nematode population is
high.
Addition of
organic amendments. Chicken manure is very effective reducing nematode egg
masses by 56%.
Use of trap crops
and antagonistic crops. Planting Tagetes erecta and Crotolaria
spectabilis in nematode infested soil is effective against the root-knot
nematode
Biological control
Paecilomyces lilacinus, a fungal egg parasite, was found effective
against root-knot attacking sweetpotato. The parasite reduced egg masses by
about 50%.
Host-resistance
There are many varieties of sweetpotato found resistant to the root-knot
nematode. Some of these are: W-86, L4-89, BPA-4 and Sinibastian, Jasper,
Jewel, Miracle, Georgia Red, Garcia Yellow and Travis. However, some populations
of M. incognita can infect even some of the resistant cultivars.
Chemical control
Several nematicide have been very effective against the root-knot nematode in
sweetpotato. Examples are Nemagon, Mocap, Dasanit, Nemacur, Furadan, Temik,
Vydate.
Evans, K, D. L. Trudgill and J. M. Webster. 1993. Plant Parasitic Nematodes
in Temperate Agriculture. University Press, Cambridge. 648 p.
Galano, C. D., R. M. Gapasin and J. L. Lim. 1996. Efficacy of Paecilomyces
lilacinus isolates for the control of root-knot nematode (Meloidogyne
incognita (Kofoid and White) Chitwood) in sweet potato. Annals of Tropical
Research 18: 4-12.
Gapasin, R. M. and R. B. Valdez. 1979. Pathogenicity of Meloidogyne
spp. and Rotylenchulus reniformis on sweet potato. Annals of Tropical
Research 1:20-26.
Gapasin, R. M. 1984. Resistance of fifty-two sweet potato (Ipomoea batatas
(L.) Lam.) cultivars to Meloidogyne incognita and M. javanica.
Annals of Tropical Research 6: 1-19.
Sasser, J. N. and C. C. Carter. 1985. An Advanced Treatise on Meloidogyne.
Vol. I: Biology and Control. North Carolina State University Graphics. 422 p.
Sasser, J. N.
1989. Plant Parasitic Nematodes: The Farmerĺs Hidden Enemy. University
Graphics, North Carolina State University, Raleigh, N. C. 115 p.
Contributed
by: Ruben Gapasin |
Taxonomy
Economic
importance
Geographical
distribution
Symptoms
Morphology
Life
cycle
Host
range
Management
References

Galls
and egg masses of root-knot nematodes on fibrous roots (left).
Galls on sweetpotato roots are often much smaller and difficult to see (W.
Martin, APS).
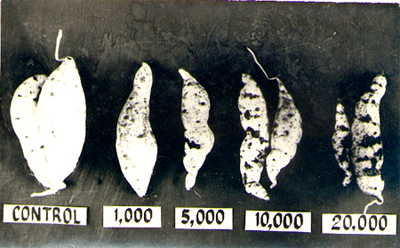
Increasing yield reduction and storage root damage with increasing
nematode population.
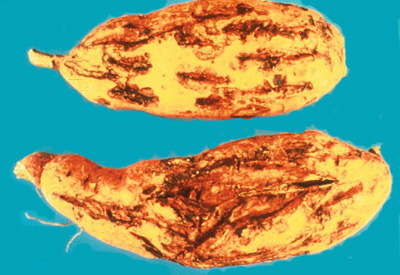
Cracking
on storage roots due to root-knot nematode (G. Lawrence, APS).
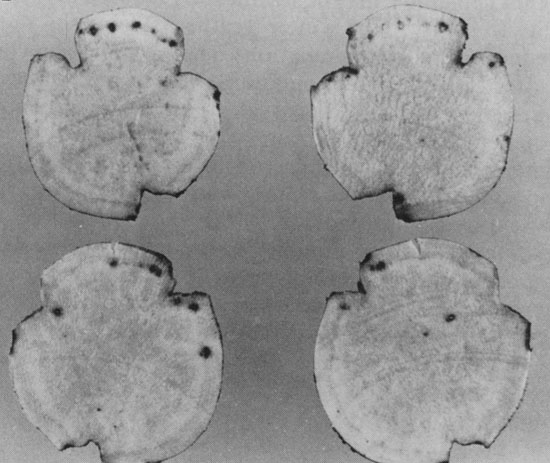
Dark spots in the outer layers of the flesh, each containing a nematode
surrounded by dark cork tissue (G. Lawrence).

Subcortical spots which were not evident until the roots were peeled (W. Martin, APS).

Small blister-like bumps are sometimes apparent on the storage root
surface, where nematodes are embedded (G. Philley)
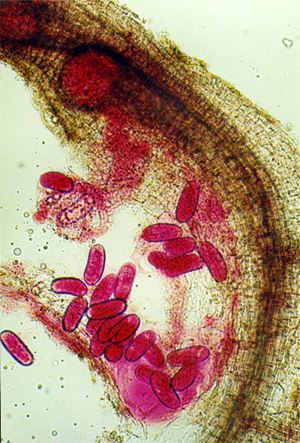
Egg
masses of M. incognita (R. Gapasin).
|

