Taxonomic Position
Cohort Gamasina
Subcohort Dermanyssiae
Superfamily Rhodacaroidea
Family: Ologamasidae Ryke
(including Gamasiphidae, Euryparasitidae)
Acugamasus Lee, Acuphis Karg, Allogamasellus Athias-Henriot, Antennolaelaps Womersley, Athiasella Lee, Caliphis Lee, Cymiphis Lee, Cyrtolaelaps Berlese, Euepicrius Womersley, Euryparasitus Oudemans, Evansellus Ryke, Gamasellevans Loots & Ryke, Gamaselliphis Ryke, Gamasiphis Berlese, Gamasiphoides Womersley, Gamasitus Womersley, Geogamasus Lee, Heterogamasus Trägårdh, Heydeniella Richters, Hiniphis Lee, Hydrogamasellus Hirschmann, Hydrogamasus Berlese, Litogamasus Lee, Laelaptiella Womersley, Lindquistoseius Genis, Loots & Ryke, Neogamsellevans Loots & Ryke, Notogamasellus Loots & Ryke, Ologamasus Berlese, Onchogamasus Womersley, Paragamasellevans Loots & Ryke, Parasitiphis Womersley, Periseius Womersley, Psammonsella Haq, Pyriphis Lee, Queenslandolaelaps Womersley, Rykellus Lee, Sessiluncus G. Canestrini, Solugamasus Lee, Tangaroellus Luxton
Diagnostic characters:
-
Female with 4 pairs of setae on a well sclerotized sternal shield
-
4 scleronoduli rarely present
-
Tibia I and genu I each with 6/4 dorsal/ ventral setae.
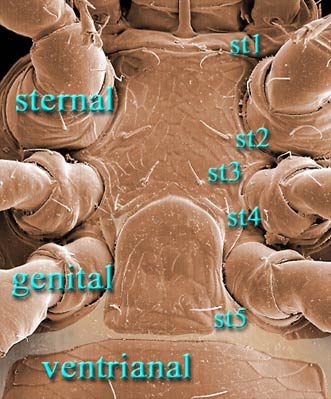
Similar taxa. The various families in the Rhodacaroidea
are usually easy to distinguish from other Mesostigmata by their entire sternal
shields with 4 pairs of setae and 6/4 dorsal/ ventral
setation of tibia I. In Rhodacaridae,
most of which are small, lightly sclerotized mites, the anterior margin of the
sternal shield is usually membranous to about the level of st1.
Digamasellidae have a highly reduced leg
chaetome and a distinctive tectum with a subdorsal median tine.
Species of both Australian Rhodacaridae (except Protogamasellopsis) and
Digamasellidae (Digamasellus) usually
have 3-4 scleronoduli, but I know of only 1
undescribed Australian ologamasid with these light refracting podonotal
structures. Some Pachylaelapidae
and Parholaspididae may have sternal
shields that 'capture' st4, but both families have highly reduced leg chaetomes.
Diagnosis. Tan, brown, orange or reddish dermanyssine
mesostigmatans. Females with separate
podonotal and opisthonotal shields, fused to smoothly holodorsal
shield, or beetle-like all encompassing armor,
rarely encased in a layer of soil; males with
holodorsal
shield. Peritremes typical running past
coxa I, sometimes chambered; peritrematal shields usually well developed. Female with large sternal shield bearing 4 pairs of setae (st1-4)
and 3 pairs of lyrifissures (stp1-3), sometimes with arms embracing
genital shield; genital shield usually mound-shaped to subrectangular, hinged at level of coxae IV, bearing 1 pair of setae, and free
from ventrianal shield. Venter covered by large ventrianal shield,
sometimes fused to peritrematal and/or dorsal shields; anal opening with 3 circumanal
setae. Tarsus I slender, sometimes
elongate, with or without claws; trochanter I with 6 setae; tibia I with 6
dorsal and 4 ventral setae; genu IV with 5 dorsal and 2 ventral setae. Chelicerae various, especially
chelate-dentate and snapping; movable digit without excrescences. Palp genu with 6 setae, palp apotele
3-tined; corniculi usually horn-like, rarely truncate and toothed; internal
male highly divided. Tritosternum
biflagellate, with columnar base.
Tectum various, but typically including an elongate median
process. Female sperm induction pores at base of
coxae IV, on coxae IV, on coxae or trochanters III, or other sites; sperm ducts
lead to unpaired, central spermathecal sack (laelapid type). Males with genital opening at base of
tritosternum in sternogenital shield; spermatodactyl simple and finger-like to
extremely complex; legs II commonly with femoral spur.
Ecology & Distribution. Ologamasids are the dominant predatory mesostigmatans in forest litter in the Southern Hemisphere, but few species have been studied in detail (see Lee 1973, 1974). Most are considered free-living predators, but species of Cyrtolaelaps and Euryparasitusare associated with mammal nests and their deutonymphs are phoretic on the nest builders. Also, deutonymphs of the aberrant genera Iphidosoma (often assigned to the Eviphididae) and Epiphis (often Rhodacaridae) are found on carabid beetles, and the former sometimes on sciarid flies.
References
Antony LM. 1987. A
phylogenetic analysis of the Rhodacaroidea (Acari : Mesostigmata). Dissertation
Abstracts International 47: 4047B.
Evans
EO & Till WM. 1979. Mesostigmatic mites of Britain and Ireland
(Chelicerata: Acari-Parasitiformes). An
introduction to their external morphology and classification. Transactions of the Zoological Society of
London 35 (2): 145-270.
Gilyarov MS & Bregatova NG
(eds) 1977. Handbook for the Identification of Soil-inhabiting Mites,
Mesostigmata. Zoological Institute
of the Academy of Sciences: Petrograd [In Russian]
Lee DC. 1966.
New species
of Ologamasus Berlese (Acari :
Rhodacaridae) from Australia and New Zealand. Records of the South Australian Museum 15: 205-235.
Lee DC. 1967.
Heterogamasus Trägårdh (Acari: Rhodacaridae), including the subgenus Evanssellus Ryke, stat. n. Records of the South Australian Museum 15: 497-512.
Lee DC. 1970.
The Rhodacaridae
(Acari : Mesostigmata); classification, external morphology and distribution of
genera. Records of the South Australian
Museum 16: 1-219.
Lee DC. 1973. Rhodacaridae (Acari : Mesostigmata) from near Adelaide,
Australia. I. Systematics.
Records of
the South
Australian Museum 16: 1-36.
Lee DC. 1973. Rhodacaridae (Acari : Mesostigmata) from near Adelaide,
Australia. II. Ecology.
Transactions of the Royal Society of South
Australia 97: 139-151.
Lee DC. 1974.
Rhodacaridae
(Acari : Mesostigmata) from near Adelaide, Australia. III. - Behaviour and
development. Acarologia 16: 21-44.
Lee, D. C. 1977.
Nomenclatural
status of Cyrtolaelapidae, Ologamasinae and Gamasellinae (Acari :
Mesostigmata). Journal of the Australian
Entomological Society 16:
297-299.
Krantz
GW. 1978. A Manual of Acarology.
OSU Bookstores: Corvallis.
Krantz GW & Ainscough
B. 1990. Mesostigmata. pp.
583-665, in DL Dindal (ed) Soil Biology Guide.
John Wiley & Sons: Brisbane.