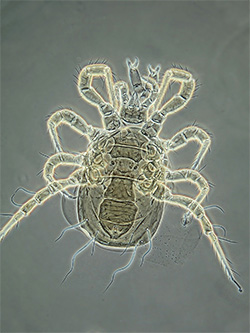
Adult Phytoseiidae - female Proprioseiopsis messor |
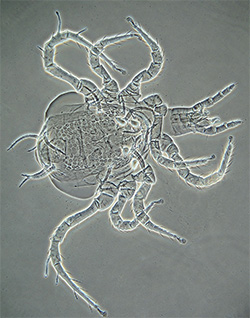
Adult Phytoseiidae - female Phytoseuis fotheringhamieae |
About Phytoseiidae
Phytoseiidae is one of the largest families of plant- and soil-inhabiting parasitiform mites (Acari) of the suborder Mesostigmata. Phytoseiidae are a cosmopolitan family, with almost 2250 species described worldwide (Moraes et al., 2005). Because of their prevalence, Phytoseiidae are often intercepted on agricultural produce, which puts them under the attention of quarantine authorities.
Phytoseiidae are small or medium-sized mites (0.2-0.58 mm in length), with rounded or egg-shaped bodies, and relatively long legs. The colour of live phytoseiids varies from colourless or pale yellow to darker shades of brown. Most species inhabit the surface of plants - leaves, bark, friuts and flowers of trees, shrubs, and herbaceous vegetation. Some Phytoseiidae prefer low-growing plant species, soil and litter.
Phytoseiidae are active predators, feeding on many groups of phytophagous mites (e.g., gall mites, spider mites), as well as thrips and other small phytophages. Many phytoseiids are generalists but a few have specific prey requirements. Many species are important biological control agents of thrips, broad mite and two-spotted mite, and several species are commercially available in New Zealand. Many phytoseiids will also eat pollen, and they are often found inside pollen-producing flowers.
In New Zealand Phytoseiidae are found on a wide range of native vegetation and on exotic plants (Collyer, 1982). Although species collections exist, the taxonomic treatment of the family in New Zealand is outdated (Collyer, 1982), and as it is, covers only two out of five NZ phytoseiid genera. The recent publication on Australian Phytoseiidae (Walter and Beard, 1997) provides the treatment and a key for a third genus - Phytoseius - and adds several distribution records for New Zealand.
Male or Female?
The identification of Phytoseiidae is based primarily on the adult females. Female Phytoseiidae can be easily recognised by the appearance of their ventral surface: females have an elongated epigynial (genital) shield between and below coxa IV, a sternal shield anterior to the genital shield, and a posterior ventrianal shield which covers the ventral and anal regions (see image below). Female Phytoseiidae posess bell-shaped, tube-shaped, or funnel-shaped spermathecae (sperm receptacles), which are usually seen between coxae of legs III and IV (spermathecae may be displaced in squashed specimens). The shape of spermathecae varies widely among species, and is an important taxonomic character.
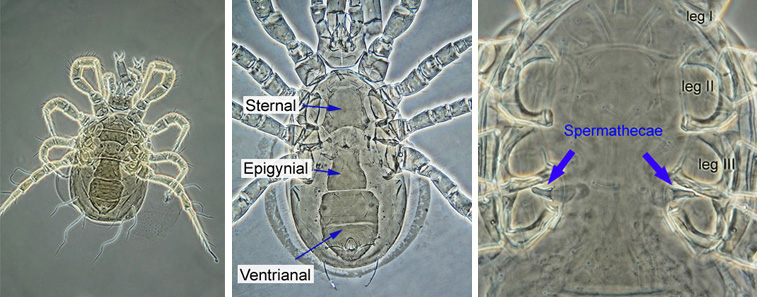 |
Males have much smaller genital opening, located between the bases of coxa I (see image below). The elongated genital shield is absent. The ventrianal shield of a male Phytoseiidae is large, triangular, and covers the entire posterior part of the ventral surface. Males have a T-shaped or a finger-shaper elongated projection, called spermatodactyl, on each chelicera - these are used for sperm transfer, and their shape is an important taxonomic character. Adult males are usually less numerous than females, and for many species the males are unknown at this time. When available, the images of adult males were included in image illustration for the species, to provide basis for identification if only males are intercepted. Immature Phytoseiidae (only sternal and small anal shields present) are unlikely to be identified correctly.
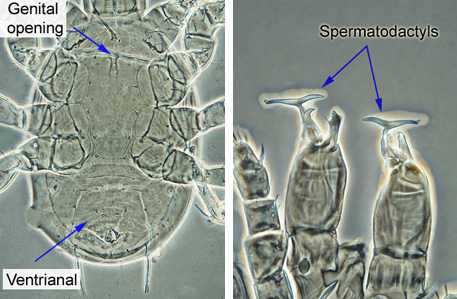 |
Sampling Methods
Apart from direct collection, two main methods can be recommended to efficiently extract Phytoseiidae from substrates they live on:
1. Plant sampling (modified from Finnamore et al., 2002) was found to extract more Phytoseiidae than other methods. In this sampling method, plant parts (leaves, branches, fruit, flowers) are selected. The plant part to be sampled (e.g., a leaf) is placed inside a polyethylene bag and is cut off. The leaf should not be disturbed before the bag is in place, since this may result in a loss of mobile Phytoseiidae. Plant parts can be collected randomly until the bag is full (6-10 leaves per site is sufficient for plants with large leaves, such as cucumber or aubergine; increase sample size if leaves are small). Collected leaves should be kept out of direct sunlight and processed as soon as possible. Samples can remain in cold storage at 4 °C for a maximum of 1 week, after which the quality of the samples declines significantly. Phytoseiidae are extracted from plant material by washing plant parts (leaves, etc.) in 70% ethanol and filtering the contents. To extract mites (Zacharda, 1988): place plant parts (leaves) in a dilute ethanol solution and agitate vigorously for 5-10 minutes, then remove the leaves. After the leaves are removed from the solution, the fluid is filtered. The filtered sediment is transferred to a vial, labeled and preserved in 75% alcohol. Ethanol samples can be stored at room temperature.
2. Berlese-Tullgren extractor can be used to separate Phytoseiidae from the litter, soil, plant debris, and other similar substrates. Until ready to extract, the soil and litter samples should be placed in plastic bags and kept in a cool, dark area at 4°C (for a maximum of 1 week). To avoid damage to fragile animals, samples need to be handled gently. The materials from which Berlese-Tullgren extractors are constructed can be extremely varied, but every Berlese-Tullgren extractor consists of a funnel, a light source, a screen, and a receptacle into which the animals fall. A litter sample is placed on the screen (for example, a sieve) at the top of the funnel. For efficient extraction, the layer of litter should not be more than 5-6 cm thick. For Phytoseiidae sampling, the mesh size of 1.5 mm would be large enough to allow the mites to pass through, but prevent most of soil and litter debris from falling into the collecting container. A low-power light bulb (10-40 Watts) positioned just above the sample is used to heat and dry the litter from above. The lower part of the funnel needs to be cooled to create a gradient of temperature and humidity. As the soil dries out from the top down, the dryness stimulates the mites to move downward and eventually fall through the sieve into a container with preservative (usually 75% alcohol). With the light bulb as a source of heat, the extraction can take 5-7 days. The Berlese-Tullgren extraction is a good method for extracting Phytoseiidae from soil and litter, but it has its own problems. First, it is an indiscriminate method that will extract all small arthropods (juvenile myriapods, insects and their larvae, mites and springtails), often in huge numbers, and the sample will require additional processing time to sort through it and to find Phytoseiidae. Second, the Berlese-Tullgren extraction is not suitable for wet substrates, or substrates such as fresh leaves, fruits, flowers, etc., as these may rot or go mouldy before the mites are extracted.
There are other good methods for collecting and extracting mites, and an extensive literature on Phytoseiidae sampling in various envionmentsis available. The literature sources should be consulted if a quantitative sampling of Phytoseiidae is to be undertaken.
Specimen preparation
The choice of a particular preservative for specimen storage and the choice of a mounting medium largely depend on the purpose of the mount. If it is desired to keep a specimen for a long time, the user should address existing literature for detailed information on preserving liquids and mounting media for mites. For routine identification (i.e., when long-term storage and preservation are not important), phytoseiid mites can be successfully stored in 70-80% ethanol (add 1-3% glycerine to protect the specimens from evaporation), and mounted in Hoyer's medium.
Hoyer's Mounting Medium
30-40 g arabic gum (clear crystals)
200 g chloral hydrate
20 ml glycerine
50 ml distilled water
Note: choral hydrate is a controlled substance.
To prepare the medium, mix arabic gum crystals and distilled water, let stand for 1-2 days until completely dissolved. Add chloral hydrate, mix and let stand for 24 hours (or until dissolved). Add glycerine. Allow any sediments to settle, and filter through glass wool. Store the Hoyer's medium in an airtight dark glass bottle. If the medium becomes too viscous, it can be diluted with small amounts of distilled water. Hoyer's medium will remain stable and provide good viewing conditions from several months to a few years.
Other clearing and mounting media (lactic acid, PVA, etc.) can be used as well. The choice of mounting medium depends on the need for long-term preservation, the user's preference, and the type of microscopy employed (for example, certain media are better for use with DIC microscopes).
Mites can be mounted on standard (75 x 25 mm) microscope slides, with 18 mm square or circular coverslips (#0 or #1). If Hoyer's medium is used, mites can be transferred from alcohol into the medium directly. When mounting the mite, make sure that it is laying flat against the slide, and that the medium fills the space under the cover slip completely. A lateral view of the extended and open chelicerae is very helpful for identification of Phytoseiidae species. When making the slide, attempt to press on the coverslip (gently, or the coverslip will break!) and to compress the mite - it often helps to flatten the mite and to extend the chelicerae. Once the slide is prepared, place it horizontally in the dessicator, or in an oven at 45-50°C until dry (at least few days). When the slide is dry, the mite will be cleared and ready for identification.
Microscope Requirements
Phytoseiidae are small mites, and a compound microscope is absolutely required to identify them. The specimens should be cleared and mounted on microscope slides (see above). Recommended setup for the compound microscope:
x10 objective lens for finding specimen on the slide;
x20 and x40 objective lenses for general identification;
x60 or x50 oil immersion objective lens for examining small details such as teeth on the chelicerae;
phase contrast condenser or Nomarski differential interference contrast (DIC) condenser are strongly recommended.
References
Collyer, E. (1982) The Phytoseiidae of New Zealand (Acarina). 1. The genera Typhlodromus and Amblyseius - keys and new species. New Zealand Journal of Zoology 9: 185-206.
Finnamore, A.T., Winchester, N.N., and Behan-Pelletier V.M. (2002) Canopy Arthropods - Branch Clipping. PROTOCOLS FOR MEASURING BIODIVERSITY: Arthropod monitoring in terrestrial ecosystems (Updated: 2002-09-30).
URL: http://www.eman-rese.ca/eman/ecotools/protocols/terrestrial/arthropods/branch.html (Accessed 2005-05-25).
Moraes, G.J. de, McMurtry, J.A., Denmark, H.A., and Campos, C.B. (2004) A revised catalog of the mite family Phytoseiidae. Zootaxa 434: 1-494.
Walter, D.E., and Beard, J.J. (1997) A Review of the Australian Phytoseiinae (Acari: Mesostigmata: Phytoseiidae). Invertebrate Taxonomy 11: 823-860.
Zacharda, M., Pultar, O., and Muska, M. (1988) Washing technique for monitoring mites in apple orchards. Experimental and Applied Acarology 5: 181-183.
