

 |
 |
 |
This is a key to the adult females of 43 families and 3 superfamilies of soil-inhabiting parasitiform mites. The Parasitiformes is one of the three major lineages of mites and the definition that I follow in this key is derived from Grandjean (1969) and Johnston (1982). Three suborders are recognized: Ixodida (ticks), Holothyrida (holothyrans), and Mesostigmata (mesostigmatans). Although some workers extend the concept of Parasitiformes by including its assumed sister group, the Opilioacarida, their logic is not convincing and I prefer the better supported, longer recognized, and more coherent definition. A name is available for the sister group Opilioacariformes + Parasitiformes (Anactinotrichida). Opilioacarids can be identified in the accompanying key to Orders, Suborders, and Cohorts of Mites in Soil.
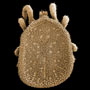
The systematics of the major parasitiform suborder, Mesostigmata, is based primarily on the adult female, and it is on that stage that accurate identifications depend. Immature mesostigmatans and adult males are unlikely to be identified correctly. Female mesostigmatans are easily recognized by their genital opening, usually found between coxa IV and covered by one or more genital shields (see details in image to your left below). Although called 'genital' (or epigynial), the shield protects only the ovipore in mesostigmatans with a secondary sperm transfer system (Dermanyssina, Discozerconidae). Females lay eggs relatively large in comparison to their body size, so the genital opening and its shield are relatively large, and in the Sejina, it may cover most of the intercoxal region.
The genital shield often bears the 5th pair of sternal setae (st5), but may be nude and variously formed (see Characters 26. Female genital shield (number, form); 29 Genital/ mesogynial shield shape). A complicating issue is the presence of accessory genital shields in early derivative taxa exhibiting the quadrigynaspid or trigynaspid condition. Adult females also usually have one or more shields anterior to the genital shield(s) called sternal shields, and these usually sport one or more of sternal setae st1-4 (see Characters 24. Sternal shields; 25. Metasternal setae (st4)). Other shields cover the ventral and anal regions (see Characters 28. Ventral shield; 33. Anal/ ventrianal shield). The female ologamasid mite above (on your left) has a large ventrianal shield, while the laelapid mite to your left below has a small anal shield.
Males, however, produce relatively small sperm packets (spermatophores) and have much smaller genital openings. Although these openings also may lie between coxae IV, they are more typically towards the anterior end of the intercoxal region and often at the base of the tritosternum (see details in the images of males to your right above and below). In the male ologamasid mite above, the genital opening is in a sternogenital (also sternitogenital) shield that bears 5 pairs of setae (st1-5). In the male laelapid mite below, however, the sternogenital shield is fused to the ventrianal shield and other elements to form a ventral shield or plate. Males also often, but not always, have modifications to their chelicerae for transferring sperm, e.g. spermatodactyls or spermatotremes.
|
|
|
This key has grown from the knowledge I’ve accumulated during 20 years of working with soil mites in the United States (mostly in California, Oregon, Colorado, New Mexico, Florida) and Australia (mostly in Victoria, New South Wales, Queensland, The Northern Territory). The key is meant to be comprehensive for the known Australian acarofauna at the time of writing (December 2000) and it includes a number of new records that are in press or in preparation. For a continually updated account of Australian mite taxa, see Bruce Halliday's Checklist of Australian Mites at the ABRS web site http://www.environment.gov.au/abrs/ABIF-Fauna/.
 |
 |
 |
While based on the known Australian fauna, the key includes groups of soil mites that have not yet (and may never be) been found in Australia, e.g. Epicriidae, Zerconoidea, Thinozerconoidea, Arctacaridae, Seiodidae, and Pyrosejidae. Zerconoidea, Thinozerconoidea, and Uropodoidea are identified only to the superfamily level. Zerconoidea (2 families) and Thinozerconoidea (3 families) are not known from Australia. Uropodoidea dominate many Australian forest litter faunas, but are very poorly known. Additionally, there is much disagreement about the family level classification, with various schemes containing from 2 to dozens of families. If anyone is looking for a group of soil mesostigmatans desperately in need of study, I highly recommend this interesting and diverse group. Another group prominent in Australian soils with varying numbers of recognized families is the Rhodacaroidea. In this key, three rhodacaroid families are treated: Rhodacaridae, Ologamasidae, and Digamasellidae. Subdivisions of the Ologamasidae (e.g. Gamasellidae, Euryparasitidae) and insect associates such as the Laelaptonyssidae are not used. For similar reasons, Leptolaelapidae is not treated.
By ‘soil-inhabiting’ I mean representatives are free-living and likely to occur in soil, litter, or associated substrates (e.g. mosses, beach wrack, compost). This key is not meant to identify mites that are parasitic or otherwise symbiotic on larger animals. Therefore, most of the families of Dermanyssoidea are excluded (except Laelapidae, many of whose species are free-living, and Macronyssidae because of their tendency to wander from their hosts). I have also excluded most of the Antennophorina that are associated with insects, myriapods, and reptiles, but have included those families that sometimes turn-up in samples of Australian soil/ litter (usually rotting logs), e.g. Diplogyniidae, Parantennulidae, Megisthanidae, and those families that appear to be essentially free-living: Celaenopsidae and Triplogyniidae.
Like any key, the accuracy of the results the user obtains will be a function of the limits of the key and the abilities and training of the user. Along with any misinterpretations or mistakes that the author has imposed on the key, a user (especially in the poorly studied tropics) is likely to encounter exceptions to currently accepted taxonomic definitions. Once an identification has been obtained, the user should always compare their specimen to the taxon images and notes: any deviation between the specimen and the diagnosis, in particular, should be a cause for concern. Please report any problems or constructive suggestions to Dave Walter at the email address below.
David Evans Walter, The University of Queensland ([email protected])
References
Grandjean F. 1936. Un acarien synthetique:
Opilioacarus segmentatus With. Bulletin,
Société d'Histoire Naturelle de l'Afrique du Nord 27: 413-444.
Grandjean F. 1969. Stases. Actinopilne. Rappel de ma classification
des Acariens en 3 Groupes majeur. Terminologies en soma.
Acarologia 16: 796-827.
Johnston DE. 1982. Mesostigmata. In: Parker, S.P. (ed.) Synopsis
and classification of living organisms. McGraw-Hill,
New York, p. 112-116.
Walter DE & Proctor HC. 1999.
Mites: Ecology, Evolution and Behaviour.
University of NSW Press, Sydney and CABI, Wallingford.
Walter DE & Proctor HC. 2000. Orders, Suborders, and Cohorts of Mites in Soil. LucID Interactive Key.
The preparation of this key was funded by the Australian Biological Resources Survey. I’d like to thank Dr Tony Orchard of ABRS for his support and encouragement. Professor G. W. Krantz of Oregon State University, Dr Hans Klompen of The Ohio State University, and Dr Evert Lindquist of Agriculture Canada all provided specimens and thoughtful comments on mesostigmatan relationships that have been of immeasurable help during the construction of this key. Dr Bruce Halliday of CSIRO Entomology kindly allowed me to use his ongoing checklist of Australian mites (soon to be on the web under GROUPS at the ABRS site http://www.environment.gov.au/abrs/ABIF-Fauna/). Catherine Harvey (CH), Dr Jennifer Beard (JB), and Owen Seeman (OS) executed the line drawings under my supervision and with my occasional and considerably less artistic help. The micrographs and scanning electron micrographs are mine and I gratefully acknowledge the help given by Dr David Merritt in setting up and using his microscope and the personnel at the Centre for Microscopy & Microanalysis at the University of Queensland for their help.
I’d also like to thank those who have helped test this key, foremost among whom is Dr Heather Proctor of Griffith University. Above all, I’d like to thank my students at The University of Queensland and the students who attended the Soil Acarology Courses at the Summer Acarology Program at The Ohio State University. It was their interest, and obvious need for a more user-friendly identification tool, that has helped to keep me going during the many hundreds of hours at the SEM, microscope and computer that it has taken to construct this key.
Dave Walter
Brisbane, December, 2000