THE WORLD SPECIES-GROUPS OF LEUCOSPIS (HYMENOPTERA: LEUCOSPIDAE)
-- 30 YEARS LATER
D. CHRISTOPHER DARLING
Department of Natural History
Royal Ontario Museum
100 Queen’s Park
Toronto, Ontario, Canada
and
Department of Zoology
University of Toronto
Toronto, Ontario Canada
M5S 1A1; [email protected]
SOPHIE CARDINAL1
Department of Biology
York University
4700 Keele Street
Toronto, Ontario Canada
M3J 1P3; [email protected]
1 Present Address: Department of Entomology, Cornell University, Ithaca, New York 14853 USA
ABSTRACT. The morphological characters used in an interactive
identification key to the world species-groups of Leucospis Fabricius, 1775 were analyzed phylogenetically to evaluate both the monophyly
and relationships of the species-groups of Leucospis. There was considerable homoplasy in the diagnositic characters and the majority
of the species-groups are only weakly supported by the cladistic analysis.
An interactive identification key was used to diagnose the species-groups
in each of the world’s major biogeographic regions. Based on these diagnoses
and the results of the cladistic analysis, a modification to Bouček’s (1974) system of species-groups is proposed: subdividing the pediculata species-group into two putatively monophyletic species-groups, the pediculata species-group (revised composition) and the micrura species-group. The use of informal species-groups, as advocated by Bouček, as opposed to poorly substantiated genera or subgenera, is a pragmatic approach
to infra-generic classification. New initiatives in taxonomy for the twenty-first
century are discussed and the suggestion is made to have Bouček’s (1974) revision be the new starting point for the taxonomy of the Leucospidae.
Key words: taxonomy, phylogenetics, species-groups, interactive key, Hymenoptera, Chalcidoidea, Leucospidae, world-wide
It has been 30 years since Zdeněk Bouček published the first and only species level revision for a major family level
taxon in the Chalcidoidea. A Revision of the Leucospidae (Hymenoptera: Chalcidoidea) of the World (Bouček 1974) was and remains a monumental work: 272 figures were included; 109 species
were recognized and keyed, 31 of which were new; 2 generic and 58 specific
and subspecific names were synonymized; and 5 new combinations were proposed.
Because of their large size and interesting color patterns these wasps attracted
considerable early attention; as a result the classification of this family
was incredibly complicated and confused, even for Chalcidoidea. Synonymy was
rampant, and resolving the nomenclature required Bouček to examine the type material for more than 150 names (including 57 holotypes)
and to designate 91 lectotypes. The vast majority of species were referred
to Leucospis and the most recent list of valid names includes 114 species. Remarkably, only
6 species of Leucospis have been described since the 1974 revision, and Bouček was an author of two of these species (references available in Noyes 2004).
Furthermore, all of the more recent authors have described their species in
the context of Bouček (1974), even when the first fossil species was described (Engel 2002).
Bouček struggled with a way to organize the more than 100 morphologically-diverse
species of Leucospis known at the time. He eventually abandoned the idea of several genera, or even
subgenera, and settled on an infra-generic classification based on species-groups
and species sola. This had the advantage of not further complicating the nomenclature
and this flexible system of classification has been recommended and used in
other complex genera of Chalcidoidea (e.g., Eupelmus Dalman, 1820 by Gibson 1995; Perilampus Latreille, 1809 by Darling 1996). The last 30 years has seen the development
of a more explicitly phylogenetic approach to taxonomy and systematics. Today’s species-groups should either be
arguably monophyletic or if not it should be clearly stated that these groupings
are ones of convenience and do not necessarily reflect the evolutionary history
of the group.
In this 30-year update to the taxonomy of Leucospis we: 1) use an interactive identification key to diagnoses the species-groups
of Leucospis by geographic region and 2) use a subset of the character states in the interactive
key to explore the phylogenetic relationships of the species of Leucospis, specifically the monophyly of the species-groups and also the relationships among
the species-groups. Finally, we briefly discuss radical and progressive initiatives
aimed at revitalizing taxonomy and suggest how these might be applied to Leucospis and the Leucospidae as recognition of a truly monumental work in chalcidoid
taxonomy.
METHODS
Lucid© (see www.lucidcentral.com ) was used to prepare an interactive identification key to the world species-groups
of Leucospis (Cardinal & Darling 2003, 2005). We prepared a Lucid Builder (ver. 2.3) database
of species, species-groups and characters using Bouček (1974) as the starting
point. Table 1 provides a current summary of the number
of species in each species-group or species sola and their geographic distributions
(see also Noyes 2004). The only modification from Bouček (1974) involves the
pediculata species-group, which is partitioned into the pediculata species-group (sensu stricto) and the micrura species group. For each of 70 species available for study, a total of 36 morphological
characters were coded along with information on geographic distribution. A
composite species-group taxon was then added using all of the character states
of the included species. This working database was then used with Lucid Player
Plus (ver. 2.2) to identify unknown specimens and to evaluate the usefulness of the species-groups.
This allowed for fine tuning the morphological characters and provided diagnoses
for species-groups and species sola. The current species-group key (16 species-groups,
4 species sola) is available on the Lucid website ( www.lucidcentral.com ) and the species-group diagnoses are derived from this interactive key.
Databases were exported using the Lucid translation wizard and the DELTA Editor
(Dallwitz et al. 1999) was used to produce a Nexus file which can be used in a variety of phylogenetics
programs. We used Winclada (ver. 1.00.08) (Nixon 1999-2002) and NONA version
2.0 (Goloboff 1999) as a matrix editor/cladogram viewer/printer and tree-searching
program, respectively. Two matrices were evaluated and the 37 identification
characters were reduced to 32 characters by removing finely differentiated
sculpture characters (n=3), an intergrading character (lower face, shape),
and the character coding for geographic distributions (See Appendix
1 and 2). Polistomorpha Westwood, 1839 and Micrapion Kriechbaumer, 1894 were coded as outgroup taxa. The monotypic genus Neleucospis Bouček, known only from the holotype female, was not included in this study,
either as an ingroup or an outgroup taxon (see Bouček 1974 for a discussion
of the affinities of this genus, which is close to Leucospis). The species matrix consisted of 70 species and the 2 outgroup taxa and was
used to determine whether or not the species-groups are “natural” groups (taxa)
or units of convenience. The species-group matrix consisted of 20 taxa (16
species-groups and 4 species sola) and was used to test both composition and
relatedness of the species-groups. All characters were treated as unordered
and unweighted and both the Heuristics and Ratchet (Nixon 1999) options of
NONA were used to find the shortest trees. Global Bremer support (Bremer 1988)
was determined using TreeRot (Sorenson 1999).
There are a number of methods for coding and sampling higher-level taxa for phylogenetic
analysis (Wiens 1998, Simmons 2001). Two were used in this study: 1) species-as-terminals
and 2) higher taxa (species-groups in this case) as terminals and coding interspecific
variation as polymorphisms (ambiguity coding). Both analyses are necessarily
preliminary and are based on a relatively few easily observable identification
characters. Our intent is not to resolve the issue of species-groups in Leucospis but rather to provide a starting point for a critical reanalysis and also to
show how Lucid databases can be used as more than interactive identification
keys.
RESULTS
Cladistic Analysis of the Species-Groups of Leucospis
The species-groups of Leucospis, Fabricius, 1775 proposed by Bouček (1974) are only weakly supported by the
phylogenetic analysis of the 70 species. Both the heuristics and ratchet analyses
produced an indeterminate number of equally parsimonius trees of length =
52 (Ci = .31, Ri = .78). Five combined heuristics analyses with max trees
= 100, number of replicates = 10, starting trees per rep = 1 (100/10/1) produced
500 trees of length 152; the same 500 trees were obtained using the parameter
values 100/10/10. And a single analysis using 1000/100/10 produced 1000 trees
of length 152. The strict consensus trees were the same in all three species-as-terminals
analyses and the topology is more conservative but otherwise similar to results
of the species-groups analysis (see Fig. 3). The following
four species-groups were monophyletic on the consensus trees and supported
by uniquely derived autapomorphies: cayennensis, affinis, texana, and pediculata (s.s.). These species-groups are all relatively small or were represented by
relatively few species in this study (3 of 9, 2 of 4, 2 of 3, and 5 of 5,
respectively). The following three species-groups were also monophyletic on
the consensus trees but supported only by characters exhibiting homoplasy:
australis (2 of 5 examined), tricolor (2 of 4), and gigas (3 of 6). There were two distinct clades of elegans group species (13 of 19 species examined) but the petiolata (5 of 7), speifera (6 of 12), hopei (4 of 9), egaia (7 of 9) and dorsigera (4 of 7) groups were not resolved.
Fig. 3 is the strict consensus tree for the species-groups analysis with interspecific
variation coded as polymorphisms. Six equally parsimonious trees (length =
88, Ci = .53, Ri = .63) were produced by the heuristic analysis. A ratchet
analysis, sampling 3 characters for 200 replicates, found 8 trees of 88 steps
and the same consensus tree was produced (Fig. 3). Fast optimization of characters
is depicted to maximize support for relationships between species-groups;
in the discussion that follows, numbers separated by a slash (/) refer to
characters and states, respectively (Appendix 1).
The species-groups with long ovipositors (dorsigera species-group and those below) are supported by 3 synapomorphies (27/3, 28/3,
29/2). However, two of these characters are almost certainly not independent
(27, 28) and there is polymorphism within the species-groups for each of these
characters. Only two clades were supported unambiguously, that is by unique
and unreversed synapomorphies, without polymorphism within the species-groups:
texana + tricolor (16/1, very small basal tooth on hind femur; 19/5 outer spur on hind tibia a
recurved hook); australis + regalis (12/2, dorsal edge of hind coxa elongate and narrow). Other possible synapomorphies
(11/1, 19/3, 23/1) are problematic because of polymorphisms within the species-groups.
Other relationships are tentatively supported by sets of homoplasious characters
(e.g., antiqua + aruina, petiolata + fuelleborniana + (texana + tricolor), and cayennensis + egaia + speifera). The overall low level of nodal support for the relationships of the species-groups
is confirmed by the Bremer analysis; only 3 sister group relationships have
Bremer support values > 1 (texana + tricolor, australis + regalis , antiqua + aruina) and in each case the value is only 2.
A review of the species-groups of Leucospis
The following is a summary of the morphological characters that can be used to
diagnose the species-groups of Leucospis found in the various biogeographic regions. Information is also provided concerning
the possible monophyly of the various species-groups by identifying putative
autapomorphies (Fig. 3). Illustrations and photographs
of all the characters and species discussed below are available in the Lucid
identification key (Cardinal & Darling 2005). The following diagnoses are
based on characters that we found particularly reliable in referring species
to species-groups. The Lucid identification key provides additional characters
(we have relied on only 25 of the 32 characters) and many of the other characters
or combinations of characters are useful when dealing with restricted sets
of species-groups, particularly when accompanied by the illustrations and
photographs in the key. Freedom to use a variety of characters in various
order and combination is one of the main advantages of interactive keys such
as Lucid (Snow & Sharp 1999).
There are no species-groups shared by the New World and the Old World. Six species-groups
are restricted to the New World and 3 have species in both the Nearctic and
Neotropical regions; the other 3 species-groups are exclusively tropical (Table
1). There is no evidence that the New World species are monophyletic (Fig.
3). Six of 10 Old World species-groups are restricted to single regions
(Table 1). The elegans and gigas species-groups are widely distributed and found in the Ethiopian, Palearctic
and Indo-Pacific regions, whereas the petiolata species-group is recorded from the Palearctic, Indo-Pacific and Australian regions,
and the dorsigera species-group is recorded from the Palearctic and Indo-Pacific regions.
NEARCTIC AND NEOTROPICAL REGIONS
Affinis species-group
Diagnosis. Propodeum with a raised callus or hump on the median area (Fig.
3, 11/2; cf. median carina present or absent in all other species-groups).
Remarks. Fig. 3 suggests that the hopei and affinis species-groups are related to the Old World gigas and elegans species-groups by the loss of the median carina on the propodeum (11/1); the
raised callus on the propodeum in the affinis species-group is a further modification.
Cayennensis species-group
Diagnosis. Mandible with semi-circular emargination (Fig.
3, 2/1; cf. triangular in all other species-groups).
Remarks. Fig. 3 weakly supports a clade consisting of
the cayennensis, egaia and speifera species-groups.
Egaia species-group
Diagnosis. Body color non-metallic but with iridescent reflections; clypeus without a median tooth; mandible with triangular emargination; occipital carina strong, distinctly extended past eye margin eye; pronotum with marginal carina.
Remarks. Fig. 3 weakly supports a clade consisting
of the cayennensis, egaia and speifera species-groups. The speifera and egaia species-groups, which together comprise over one-half of all New World species
(Table 1) are only distinguished by the presence or
absence of the marginal carina on the pronotum. All other characters are either
the same or overlapping and both species-groups are highly polymorphic for
many other character states (Appendix 2). Establishing
the monophyly of the speifera and egaia species-groups or a revised species-groups delineation is a high priority for
future research.
Hopei species-group
Diagnosis. Body color non-metallic, without iridescent reflections; hind tibia extended, adtarsal margin concave, with indistinct outer spur.
Remarks. There are no defining apomorphies identified in Fig.
3. The hopei species-group shares a derived configuration of the hind tibia with the Ethiopian
gigas and elegans species-groups (the outer tibial spur is indistinct) and may be the New World
representative of this lineage.
Speifera species-group
Diagnosis. Body color non-metallic but with iridescent reflections; clypeus without a median tooth; mandible with triangular emargination; hind tibia apex truncate to slightly extended; pronotum without carinae.
Remarks. Fig. 3 weakly supports a clade consisting of
the cayennensis, egaia and speifera species-groups; see also remarks for the egaia species-group.
Texana species-group
Diagnosis. Body color non-metallic, without iridescent reflections; basal tooth on hind femur very small (16/1; cf. long in all other species-groups); outer spur of hind tibia a recurved hook.
Remarks. The reduced basal tooth and highly modified outer tibial spur is shared
only with the Ethiopian tricolor species-group (Fig. 3). See also remarks for the Ethiopian
fuelleborniana species-group.
PALEARCTIC REGION
Four species-groups occur in the Mediteranean and West Palearctic regions (sensu Bouček 1974). All are found in other regions of the Old World tropics and only the dorsigera species-group is maximally speciose in the Palearctic region.
Dorsigera species-group
Diagnosis. Basal tooth on hind femur at least as large as femoral teeth; marginal and premarginal carinae on pronotum distinct but not strongly recurved (cf. elegans species-group); propodeum short, not distinctly longer than dorsellum.
Remarks. Leucospis japonica Walker, 1871 is widely distributed in temperate Asia and its range extends as far south as northern India and Taiwan (Bouček 1974). The later is the only record of the dorsigera species-group from the Indo-Pacific region.
Elegans species-group
Diagnosis. Pronotum with discal, marginal, and premarginal carinae distinct and strongly angulate and strongly recurved toward mesoscutum.
Remarks. This species-group is well represented in the Ethiopian and Indo-Pacific regions (q.v.).
Gigas species-group
Diagnosis. First and second basal femoral teeth oriented at an angle relative to distal teeth; distal femoral teeth parallel-sided, apices rounded; pronotum without discal carina; T5 short, less than 4 times the length of T4; ovipositor sheaths long, reaching the base of T1.
Petiolata species-group
Diagnosis. Basal tooth of hind femur distinct but smaller than femoral teeth; pronotum without discal carina; T5 long, at least 4 times the length of T4; propodeum long, about twice length of dorsellum.
ETHIOPIAN REGION
Four species-groups and 2 species sola are present in sub-saharan Africa; two
of the species-groups, fuelleborniana and tricolor, are endemic (Table 1). All species are non-metallic in
colour without iridescent reflections. The sister genus of Leucospis, Micrapion (Fig. 3), is restricted to the Ethiopian region.
Elegans species-group
Diagnosis. Pronotum with discal, marginal, and premarginal carinae distinct and strongly angulate and strongly recurved toward mesoscutum.
Remarks. This species-group is also well represented in the Indo-Pacific region (q.v.).
Fuelleborniana species-group
Diagnosis. Mesoscutum with cross carina (Fig. 3, 8/2;
cf. absent in all other species); femoral teeth arranged in a line; distal
femoral teeth triangular, apices pointed; T5 long, at least 4 times the length
of T4.
Remarks. Fig. 3 supports the contention of Bouček (1974)
that the petiolata and fuelleborniana species-groups are closely related and could be regarded as a single species-group;
the later is distinguished by the apomorphic configuration of the mesoscutum.
Both species-groups also share a highly modified hind tibia with the outer
spur represented as a short pointed nub. Consistent with this hypothesis is
the absence of the petiolata species-group from the Ethiopian region (Table 1). These
two species-groups have a sister group relationship with texana + tricolor species-groups (Fig. 3) suggesting that the New World
texana species-group has Ethiopian affinities.
Gigas species-group
Diagnosis. First and second basal femoral teeth oriented at an angle relative
to distal teeth; distal femoral teeth parallel-sided, apices rounded; ovipositor
sheaths long, reaching the base of T1; stigmal vein bilobed, stigma and uncus
distinct (as in Fig. 1); T5 short, less than 4
times the length of T4.
Tricolor species-group
Diagnosis. Basal tooth on hind femur very small; outer spur of hind tibia a recurved hook.
Remarks. See discussion of the New World texana species-group.
Holubi species sola
Remarks. This is the only Ethiopian species with the stigmal completely reduced
(i.e., stigma and uncus contiguous, as in Fig. 2).
This character state is diagnostic for the Indo-Pacific micrura species-group and interpreted as a convergence (see also remarks under micrura species-group).
Namibica species sola
Remarks. This is the only described species with the posterior margin of the
fourth gastral tergite (T4) angulate and produced toward the apex of the ovipositor
furrow on T5. This character is diagnostic for Micrapion and this similarity was noted by Bouček (1974). Also, L. namibica Bouček, 1974 is the only Ethiopian species with a tooth on the hind coxa, which
is also found in species of Micrapion. Fig. 3 suggests that these similarities are a result
of convergence. Undescribed species closely related to L. namibica have recently been collected in South Africa and Madagascar (California Academy
of Sciences) and this material and recently collected specimens of Micrapion will allow a closer study of relationships of Leucospis namibica and Micrapion.
AUSTRALIAN REGION
This region comprises Australia, exclusive of the wet tropics of Queensland, including New Zealand, Lord Howe and Norfolk islands (sensu Gressitt 1956). Only seven species and two species-groups are represented, the widely distributed petiolata species-group and the endemic australis species-group.
Australis species-group
Diagnosis. Basal tooth of hind femur at least as long as femoral teeth; dorsellum bidentate.
Remarks. The australis species-group is interpreted as the sister group of regalis species sola (Fig. 3). This sister group relationship
is supported by the shape of the hind coxa (12/2; with dorsal edge elongate
and narrowed) and the presence of a tooth on the hind coxa (13/3). The first
character is also found in a single species of the petiolata species-group and the second also characterizes the affinis species-group. There are sporadic occurrences of a tooth on the hind coxa in
other species-groups (e.g., the New World egaia and speifera species-groups, petiolata species-group).
Petiolata species-group
Diagnosis. Basal tooth of hind femur distinct but smaller than femoral teeth; dorsellum transverse, apex acarinate and rounded.
Remarks. See discussion under the Indo-Pacific region and of the Ethiopian fuelleborniana species-group.
INDO-PACIFIC REGION
The Indo-Pacific region (sensu Schuh & Stonedahl 1986 and Gressitt 1956) comprises
the tropical zones of the Asiatic and Australian regions (sensu Bouček 1974)
and includes New Caledonia. This region has the highest diversity of species-groups
(7) and two species sola. We have partitioned Bouček’s pediculata species-group into two groups, the pediculata species-group (revised composition) and the micrura species-group. There are no characters uniting these species (Fig.
3) but as will be discussed, both groups are easily distinguished and
arguably monophyletic on the basis of morphological characters. This realignment
of species will facilitate the description of new species in both species-groups
and will allow phylogenetic analyses of species relationships and subsequent
historical biogeographic analyses of these clades (Darling in prep.).
Dorsigera species-group
Diagnosis. Basal tooth on hind femur at least as large as femoral teeth; discal carina absent; marginal and premarginal carinae on pronotum distinct but not strongly recurved (cf. elegans species-group); propodeum short, not distinctly longer than dorsellum.
Remarks. Leucospis japonica is the only Palearctic species that extends its range south into the Indo-Pacific region. Long series of specimens have been collected in Taiwan (data in Bouček 1974).
Gigas species-group
Diagnosis. Basal tooth on hind femur distinct but smaller than femoral teeth; first and second basal femoral teeth oriented at an angle relative to distal teeth; distal femoral teeth parallel-sided, apices rounded; discal carina absent.
Remarks. Two species are recorded for this region: L. darjilingensis Mani, 1937is known only from the holotype (North India) and L. histrio Maindron, 1878 is distributed across the region, from India to the Solomon Islands.
Elegans species-group
Diagnosis. Pronotum with discal, marginal, and premarginal carinae distinct and strongly angulate and strongly recurved toward mesoscutum; hind femur robust, length at most twice maximum width.
Remarks. The elegans species-group is equally diverse in both the Ethiopian and Indo-Pacific regions.
The consensus tree of the species data matrix recognized two clades for the
elegans group species. One contained 3 species (Palearctic and Ethiopian) and the other
contained all of the Indo-Pacific species, 2 Ethiopian species and L. holubi Bouček, 1974. These two groups differ primarily in the structure of the dorsellum.
Bouček (1974) noted differences in the shape of the middle teeth of the hind
femur in the Ethiopian and Indo-Pacific species (character not used in this
study). These characters and the high degree of polymorphism in Appendix
2 question the monophyly of this species-group.
Petiolata species-group
Diagnosis. Body color non-metallic without iridescent reflections; apex of hind tibia extended into a finger-like projection, the outer tibial spur a short pointed nub; femoral teeth arranged in a line; distal femoral teeth triangular, apices pointed.
Remarks. Although speciose and widely distributed (Japan, throughout the Indo-Pacific
region, Australia to the Solomon islands) this species-group shows low levels
of polymorphism for the characters coded in Appendix
2. However, the cladistic analysis provides no support for the monophyly
of this species group. See also discussion of the Ethiopian fuelleborniana species-group.
Pediculata species-group (Fig. 1)
This and the following species-group are segregates of the pediculata-group (sensu Bouček 1974). Bouček provided a list of characters for his more
inclusive species-group and most of these were included in the interactive
identification key and also in the cladistic analysis. There is no support
for the monophyly of a combined group (Fig. 3). One
possible unifying character, used by Bouček in his identification key, is
the dark apical spot on the apex of the forewing. All species have a darker
apical spot on the forewing except L. giraulti Bouček, 1974 (Queensland, Australia). However, a dark apical spot is also found
in some species of Micrapion.
Diagnosis. Hind femur with comb of 25 or more very small teeth (Fig.
1); stigmal vein bilobed, stigma and uncus distinct (Fig.
1).
Remarks. Bouček (1974) used the comb-like teeth on the hind femur to group the
described species in his identification key (p. 159). This configuration of
the femur is unique in Leucospis and interpreted as a synapomorphy in Fig. 3 (15/2). We
restrict membership in this species group to Leucospis pediculata Guérin-Méneville, 1844; L. calligastri (Ferrière 1938); L. giraulti Bouček, 1974; L. pyriformis (Weld, 1922), and L. williamsi Bouček, 1974. See Noyes (2004) for species synonymies. There are also two undescribed
species (Darling, in prep.), from Indonesia (Sulawesi, Fig.
1) and the Philippines.
Micrura species-group (Fig. 2)
We newly establish this species-group as the second segregate of the pediculata-group (sensu Bouček 1974).
Diagnosis. Hind femur with less than 25 larger, triangular teeth (Fig. 2); stigmal
vein with stigma and uncus contiguous (Fig. 2);
Remarks. We assign to the micrura species-group Leucospis micrura Schletterer, 1890; L. bakeri Crawford, 1915; L. globigera Bouček, 1974; L. lankana Bouček & Narendran, 1981; L. maculata Weld, 1922; and L. nambui Habu, 1977. See Noyes (2004) for species synonymies. There are also seven undescribed
species (Darling, in prep.) from Thailand (Fig. 2),
Malaysia, and Indonesia. The only other species in the genus with the apomorphic
configuration of the stigmal vein is the Ethiopian speces sola L. holubi. A similar configuration of the stigmal vein was illustrated in Bouček (1974)
for Micrapion richardsi Bouček , 1974 (his fig. 264). All other species of Micrapion examined have the stigma and uncus separate suggesting that a contiguous stigma
and uncus is apomorphic in Leucospis.
Aruina species-group and antiqua species sola
Diagnosis. Body color non-metallic but with iridescent reflections, prominent on vertex; hind coxa very elongate, dorsal edge flat or convex with broad dorsal side.
Remarks. Bouček (1974) recognized both the distinctness of the 3 included species
of his aruina species-group and also proposed the sister group relationship with L. antiqua Walker, 1860 suggested in Fig. 3. The only outstanding question is whether all these should
be treated as a single species-group. The absence of the discal carina in
the aruina group species was noted and emphasized by Bouček but there are only a total
of 5 morphological differences in the characters listed in Appendix
2. These concern the sculpture of the dorsellum, the length of a hind
tibial carina, the occipital carina and the length of the propodeum. These
are all characters that vary within at least some species-groups. Leucospis antiqua is the only species of Leucospis with a very narrow hind femur, the length about 3 times the width. It is also
the only species outside the elegans species-group to have the pronotal carinae distinct, strongly angulate and strongly
recurved toward mesoscutum. This species is restricted to New Caledonia and
surrounding islands, a region famous for harbouring phylogenetically-distinct
and basal lineages (Chazeau 1993, Lowry 1998). Bouček further suggested that
L. antiqua and L. aruina group species are the most primitive in the genus Leucospis but did not elaborate further. The extremely elongate coxa is shared with the
basal genus of the Leucospidae, Polistomorpha but all other shared similarities are absences of characters that define the
other species-groups. Fig. 3 does not reject a sister group relationship of
the aruina species-group + L. antiqua with the rest of the genus.
Regalis species sola
This is the only species of Leucospis that is completely metallic in color, with vibrant iridescent reflections. An affinity with the australis species-group is suggested in Fig. 3. See discussion of the australis species-group.
CONCLUSIONS
There are two general conclusions from this re-examination of Bouček’s (1974) treatment of Leucospis. Firstly, the revision has stood the test of time and 30 years later remains
the starting point for the study of the taxonomy of these wasps. Secondly,
less than one-half (7 of 16) of the species-groups proposed by Bouček are demonstrably monophyletic based on the currently available suite of morphological
characters. This is not to say that the species-groups are not of utilitarian
value but at present it would be inappropriate to focus interpretive studies
of host associations or biogeography on these unsupported species-groups.
This is one reason for partitioning the pediculata species-group (sensu Bouček 1974) into putatively monophyletic species-groups.
There are other ways to manipulate the data in Appendix
2 to possibly improve the resolution of the cladogram (e.g., increase
the consistency index, resolve polytomies). For example, Wiens (1998) has
shown with simulation studies that majority coding (removing polymorphisms
by using the most common state) improves the accuracy of cladograms. Simmons
(2001) suggests abandoning the use of ambiguity coding and using exemplars
providing that there is an adequate breath of taxon and character sampling
but notes that data sets can become to large to be effectively analyzed. This
is the approach we used with the species matrix (70 taxa) and as predicted
the analysis became computationally challenging. Another possible source of
error is the use of Polistomorpha as the outgroup taxon. This genus is highly apomorphic in a number of characters
(e.g., mouthparts) and it might be instructive to use a variety of basal Chalcididae
(Wijesekara 1997) to determine character polarity in Leucospis.
This phylogenetic analysis serves to highlight Bouček’s appreciation of the high degree of variability in gross morphological characters
in the genus (p. 30-34, 101, 155 and in the keys to species). Many of the
characters are derived from the hind legs and there are correlated suites
of characters on the femur and tibia, and perhaps also on the coxa. It is
almost certain that there is a functional suite of characters involved with
the hind legs. Similar leg modifications are also found in the Chalcididae
(Wijesekara 1997), Torymidae (Grissell 1995), and Pteromalidae – Cleonyminae
(Gibson 2003) which supports the hypothesis of convergent modifications. This
analysis has shown that these characters alone will not be able to resolve
the phylogenetic relationships of the species of Leucospis. As for most families of Chalcidoidea, we need to develop novel and detailed
character systems based on comparative morphology rather than simply throwing
up our hands and grasping at DNA straws.
DISCUSSION
These is much talk today about how to rejuvenate taxonomy, a discipline both
steeped in formalized rules and of unparalleled relevance for the preservation
of biodiversity in the twenty-first century (e.g., Godfray & Knapp 2004).
One radical approach is to fully embrace twenty-first century information
science and the Internet to move forward expeditiously to catalogue the diversity
of life rather than conducting business as usual. Godfray (2002) has suggested
that the taxonomy of particular groups be based on a “first web revision”
and that after adoption by the scientific community all future work on the
group would start with that revision. The taxonomy of the group would, in Godfray’s words, “at a stroke be liberated from nineteenth-century
descriptions and potentially undiscovered synonyms”. For Leucospidae, Bouček (1974) would only need to be updated and converted electronically to be that
“first web revision”. Perhaps we should even take this one step further and
suggest that this “first web revision” be the new starting point for the nomenclature
of the Leucospidae. If the 60 synonymies and 91 lectotypes established in
Bouček (1974) were accepted by the chalcidological community the 114 valid names
(Noyes 2004) would become the only available names for Leucospis. The “first web revision” would effectively become the “New Linnaeus 1758” for
this family. The International Code of Zoological Nomenclature (ICZN 1999)
has opened the door for such initiatives (Scoble 2004). Article 79 states
that an international body of zoologists may propose that the Commission adopt
for a major taxonomic group a Part of the List of Available Names in Zoology. But why restrict this list to available names? If future taxonomists working
in the group only had to consider the 114 valid names of Leucospis, all of which would be described and illustrated in the “first web revision”,
the descriptions of new species could be expedited. This would ultimately
lead to not only a better understanding of the alpha-diversity of Leucospis but also to a more robust phylogeny. It would then be possible to answer questions
about the evolution of host associations, mimicry, and speciation in Leucospis. We hasten to add that Article 79 provides checks and balances and that the
establishment of a new starting point for the taxonomy of any group should
not be taken lightly and without careful review by the scientific community.
Bouček’s (1974) world revision of the family Leucospidae stands as a model in the
Chalcidoidea. This taxonomy has stood the test of time and it has and will
remain the starting point for the study of these wasps into the future. Regardless of whether or
not the taxonomic decisions become fixed in the future by a decision of the
International Commission on Zoological Nomenclature, this publication or its
critical components, the descriptions and illustrations, should be made available
electronically on the web. It would be a fitting tribute to the outstanding
career of Zdeněk Bouček to have his revision of the Leucospidae as the first web-based revision of
a family of Chalcidoidea.
ACKNOWLEDGEMENTS
This paper is dedicated to Zdeněk Bouček on the occasion of his 80th birthday. He has been an inspiration to the senior author for the past forty
years and it has been both a pleasure and a challenge to muddle along in his
footsteps. Assistance with the numerical cladistic analyses was provided by
Andy Bennett and Mike Spironello. This research was supported by operating
grants from the Natural Sciences and Engineering Research Council of Canada
to D. C. Darling and L. Packer.
REFERENCES
Bouček Z. 1974: A revision of the Leucospidae (Hymenoptera: Chalcidoidea) of the
World. Bulletin of the British Museum of Natural History Entomology Supplement 23: 1-241.
Bremer K. 1988: The limits of amino acid sequence data in angiosperm phylogenetic
reconstruction. Evolution 42: 795-803.
Cardinal S. & Darling D. C. 2003: Interactive identification key to the world
species-groups of Leucospis (Hymenoptera: Leucospidae). Chalcid Forum 25: 10-11.
Cardinal S. & Darling D. C. 2005: Key to the world species-groups of Leucospis
(Hymenoptera: Leucospidae). www.lucidcentral.com
Chazeau J. 1993: Research on New Caledonian Terrestrial Fauna: Achievements and
Prospects. Biodiversity Letters 1: 123-129.
Dallwitz M. J., Paine T. A. & Zurcher E. J. 1999: User’s guide to the DELTA Editor.
http://biodiversity.uno.edu/delta/
Darling D. C. 1996: Generic concepts in the Perilampidae (Hymenoptera: Chalcidoidea):
an assessment of recently proposed genera. Journal of Hymenoptera Research 5: 100-130.
Engel M. S. 2002: The first leucospid wasp from the fossil record (Hymenoptera:
Leucospidae). Journal of Natural History 36: 435-442.
Gibson G. A. P. 1995: Parasitic wasps of the subfamily Eupelminae: classification
and revision of world genera (Hymenoptera: Chalcidoidea: Eupelmidae). Memoirs on Entomology, International 5: 1-421.
Gibson G. A. P. 2003: Phylogenetics and classification of Cleonyminae (Hymenoptera:
Chalcidoidea; Pteromalidae). Memoirs on Entomology, International 16: 1-339.
Godfray H. C. J. 2002: Challenges for taxonomy. Nature 417: 17-19.
Godfray H. C. J. & Knapp S. (eds) 2004. Taxonomy for the twenty-first century.
Philosophical Transactions of the Royal Society B 359(1444): 557-739.
Goloboff P. A. 1999: NONA, version 2.0. Program and documentation. Argentina,
Tucuman: Published by the author.
Gressitt J. L. 1956: Some distribution patterns of Pacific Island faunae. Systematic Zoology 5: 11-32.
Grissell E. E. 1995: Toryminae (Hymenoptera: Chalcidoidea: Torymidae) a redefinition,
generic classification and annotated world catalog of species. Memoirs on Entomology, International 2: 1-470.
ICZN 1999: International Code of Zoological Nomenclature, Fourth Edition. International
Trust for Zoological Nomenclature.
Lowry P. P. II. 1998: Diversity, endemism, and extinction in the flora of New
Caledonia: a review. Pp.: 181-206. In: Peng C. I. and P. P. Lowry II (eds):
Rare, Threatened, and Endangered Floras of Asia and the Pacific Rim. Tapei: Inst. Botany, Acad. Sinica Monogr. 16, 432 pp.
.
Nixon K. 1999: The parsimony ratchet: a new method for rapid parsimony analysis.
Cladistics 15: 407-414.
Nixon K. 1999-2002: WinClada version 1.00.08. Ithaca, New York: published by
the author.
Noyes J. S. 2004: Universal Chalcidoidea Database. The Natural History Museum
London. http://www.nhm.ac.uk/entomology/chalcidoids , accessed July 16, 2004.
Schuh R. T. & Stonedahl G. M. 1986: Historical Biogeography in the Indo-Pacific:
a cladistic approach. Cladistics 2: 337-355.
Scoble M. J. 2004: Unitary or unified taxonomy? Philosophical Transactions of the Royal Society B 359(1444): 699 - 710
Simmons N. B. 2001: Misleading results from the use of ambiguity coding to score
polymorphism in higher-level taxa. Systematic Biology 50: 613-620.
Snow N. & Sharp D. 1999: LucID Professional for Windows©: contemporary identification
tools. Systematic Biology 48: 828-830.
Sorenson M. D. 1999: TreeRot, version 2. Boston, MA: Boston University,.
Wiens, J. J. 1998: The accuracy of methods for coding and sampling higher-level
taxa for phylogenetic analysis: a simulation study. Systematic Biology 47: 397-413.
Wijesekara F. A. W. 1997: Phylogeny of Chalcididae (Insecta: Hymenoptera) and
its congruence with contemporary hierarchical classification. Contributions of the American Entomological Institution 29(3): 1-61.
FIGURES
Figs. 1, 2. Habitus drawings. 1. Undescribed species
in the Leucospis pediculata species-group from Indonesia. 2. Undescribed species in the Leucospis micrura species-group from Thailand.
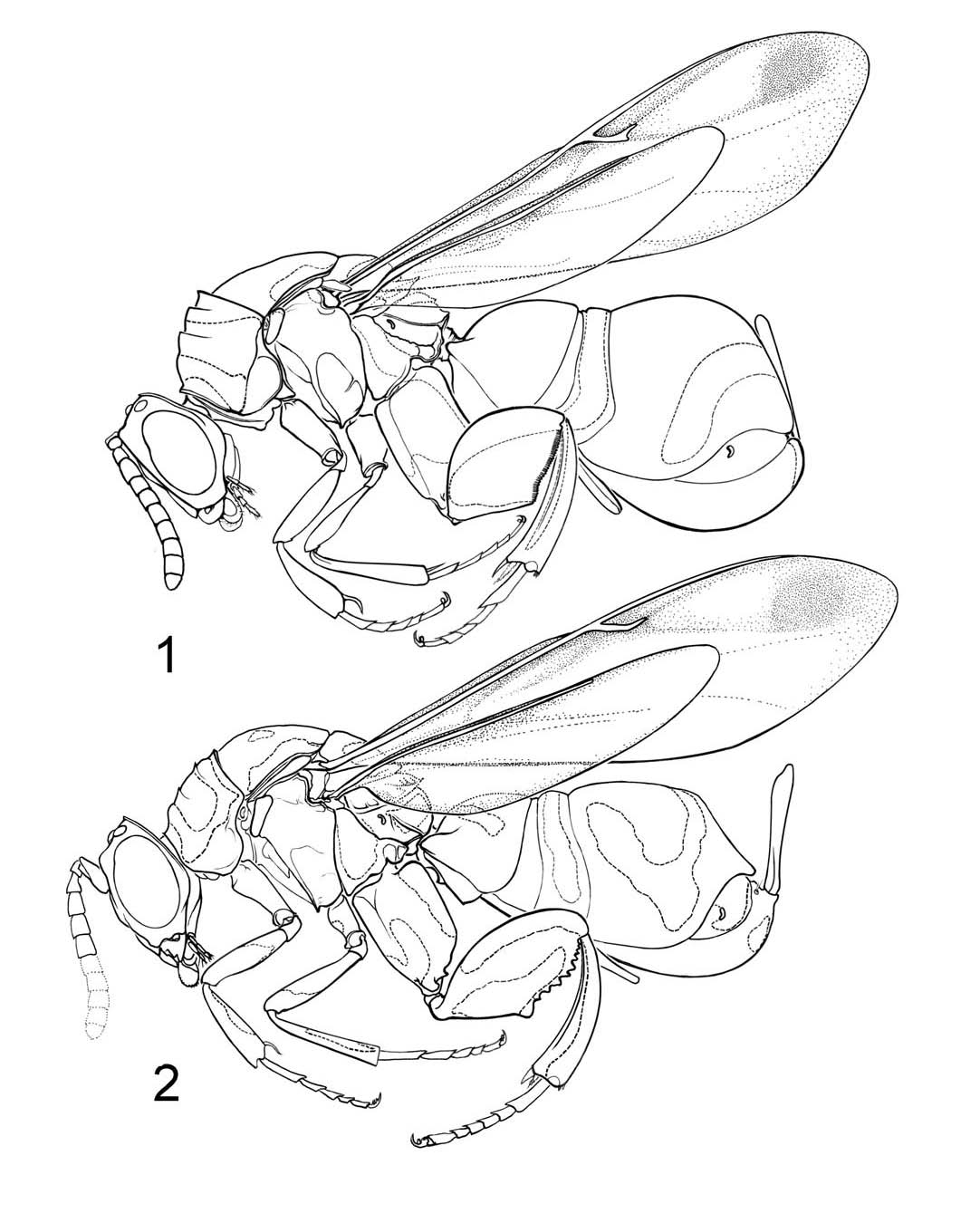
Fig. 3. The phylogenetic relationships of species-groups
and species sola of Leucospis. Consensus tree of 6 equally parsimonious trees (length = 88, Ci = .53, Ri =
.63) produced by a heuristic analysis of the data matrix in Appendix 2. See
text for discussion. Closed circles, unique and unreversed changes, synapomorphies
and autapomorphies; open circles, homoplasious changes, convergences and reversals.
Biogeographic regions indicated in brackets: NW, New World; Pa, Palearctic;
Eth, Ethiopian; I-P, Indo-Pacific; Au, Australian.
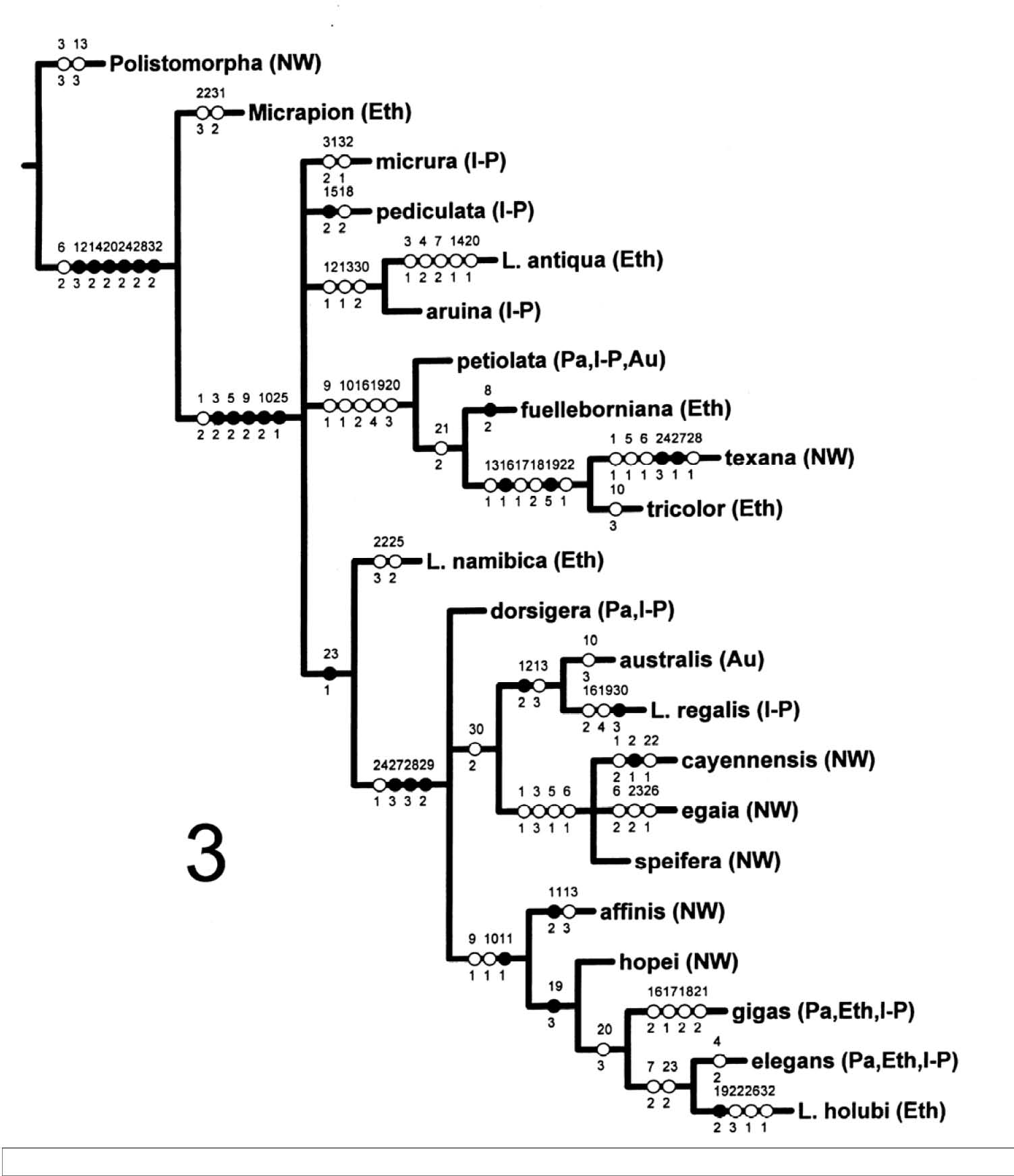
Table 1. Geographic distribution of species-groups and species
sola and number of species of Leucospis Fabricius. Biogeographic regions follow Schuh & Stonedahl (1986) for the Old
World tropics; [ ] indicates a fossil species; total number of species: 114;
note: some species are found in >1 biogeographic region.
|
|
|
|
|
|
|
|
|
|
Species group
|
Nearctic
|
Neotropical
|
Ethiopian
|
Palearctic
|
Indo-Pacific
|
Australian
|
Subtotals
|
|
affinis
|
1
|
4
|
|
|
|
|
4
|
|
antiqua (species sola)
|
|
|
|
|
1
|
|
1
|
|
aruina
|
|
|
|
|
3
|
|
3
|
|
australis
|
|
|
|
|
|
5
|
5
|
|
cayennensis
|
|
9
|
|
|
|
|
9
|
|
dorsigera
|
|
|
|
7
|
1
|
|
7
|
|
egaia
|
|
9
|
|
|
|
|
9
|
|
elegans
|
|
|
8
|
2
|
9
|
|
19
|
|
fuelleborniana
|
|
|
2
|
|
|
|
2
|
|
gigas
|
|
|
2
|
2
|
2
|
|
6
|
|
holubi (species sola)
|
|
|
1
|
|
|
|
1
|
|
hopei
|
|
9
|
|
|
|
|
9
|
|
micrura
|
|
|
|
|
6
|
|
6
|
|
namibica (species sola)
|
|
|
1
|
|
|
|
1
|
|
pediculata
|
|
|
|
|
5
|
|
5
|
|
petiolata
|
|
|
|
1
|
5
|
2
|
7
|
|
regalis (species sola)
|
|
|
|
|
1
|
|
1
|
|
speifera
|
2
|
11[1]
|
|
|
|
|
11[1]
|
|
texana
|
2
|
2
|
|
|
|
|
3
|
|
tricolor
|
|
|
4
|
|
|
|
4
|
|
|
|
|
|
|
|
|
|
|
Number of species
|
5
|
44[1]
|
18
|
12
|
33
|
7
|
113[1]
|
|
Number of species-groups
|
3
|
6
|
6
|
4
|
9
|
2
|
|
|
|
|
|
|
|
|
|
|
Appendix 1. Character descriptions. All characters are
unordered. For more complete descriptions and illustrations of the various
characters and states see Cardinal & Darling 2005).
1. Clypeus: without median tooth = 1; with median tooth = 2.
2. Mandibular emargination: semicircular = 1; triangular = 2.
3. Occipital carina: indistinct = 1; distinct = 2; strong = 3.
4. Discal carina: absent = 1; present = 2.
5. Premarginal carina: absent = 1; present = 2.
6. Marginal carina: absent = 1; present = 2.
7. Pronotal carinae (if present): transverse = 1; recurved, subangulately raised = 2.
8. Mesoscutum, cross carina: absent = 1; present = 2.
9. Dorsellum, apex: acarinate = 1; carinate = 2.
10. Dorsellum, shape: transverse, apex rounded = 1; produced, apex rounded or emarginate = 2; produced, apex strongly bidentate = 3.
11. Propodeum, median area: carina absent = 1; with a distinct raised callus = 2; carina present = 3.
12. Hind coxa, shape: very elongate, with broad dorsal side = 1; elongate, with narrow dorsal side = 2; quadrate, with narrow dorsal side = 3.
13. Hind coxa, dorso-posterior edge: smooth = 1; carinate or with serrate carina = 2; with distinct tooth = 3.
14. Hind femur, shape: slender = 1; robust = 2.
15. Hind femur, teeth: irregular, less than 25 = 1; comb-like, more than 24 = 2.
16. Hind femur, basal tooth: absent or extremely small = 1; smaller than femoral teeth = 2; at least as large as femoral teeth = 3.
17. Hind femur, orientation of basal femoral teeth: second and often the first at an angle relative to the distal teeth = 1; all teeth linear = 2.
18. Hind femur, shape of distal femoral teeth: triangular = 1; parallel-sided = 2.
19. Hind tibia, apex: truncate to slightly extended = 1; extended, adtarsal margin concave, with distinct outer spur = 2; extended, adtarsal margin concave, with indistinct outer spur = 3; extended, adtarsal margin straight or slightly convex or concave, outer spur a pointed nub = 4; extended, adtarsal margin straight or slightly convex or concave, outer spur a recurved hook = 5.
20. Hind tibia, length of outer carina: short, less than 1/2 length of tibia = 1; intermediate, 1/2 to 3/4 length of tibia = 2; long, greater than 3/4 length of tibia = 3.
21. Hind tibia, shape of outer carina: straight = 1; wavy = 2.
22. T1, shape (female): transverse = 1; quadrate = 2; elongate = 3.
23. T5, length (female): short = 1; long = 2.
24. T5, shape in lateral view (female): flat = 1; angulate, T6 and epipygium visible in dorsal view = 2; expanded, T6 and epipygium not visible in dorsal view = 3.
25. T4, posterior margin (female): rounded = 1; angulate = 2.
26. Ovipositor furrow, shape (female): divergent = 1; parallel-sided = 2.
27. Ovipositor furrow, length (female): short or absent = 1; intermediate = 2; long = 3.
28. Ovipositor sheaths, length (female): short = 1; intermediate = 2; long = 3.
29. Propodeum, length: short = 1; intermediate = 2; long = 3.
30. Body coloration: completely non-metallic = 1; non-metallic but with iridescent reflections on some structures = 2; completely metallic = 3.
31. Forewing: without infuscate apical spot = 1; with infuscate apical spot = 2.
32. Stigmal vein, shape: stigma and uncus contiguous = 1; bilobed, stigma and uncus distinct = 2.

