

Home
A Lucid Key works by progressively eliminating taxa from an identification process until, optimally, only one option remains. The user chooses a state within a selected feature, and the key discards those taxa that do not have that state, and retains those that do have that state.
Conversely, some families may be retained not because they possess a certain feature, but because we have determined that it is possible that a user may misinterpret a feature. For example, stemmata are sometimes difficult to see and may be scored incorrectly, or an eversible glandular process may be hidden and can only be identified by the presence of a slit, which is easily overlooked. We have attempted to allow for such errors. Nonetheless every effort should be made by the user to ensure correct scoring of features for optimal use of the key.
Sometimes it may be difficult to separate two or more closely related families, but this should still be considered a positive result, as the remaining families can then be compared more thoroughly, using other resources. For taxa of northern Australian biosecurity concern, Fact Sheets provide further identification information. More Fact Sheets on additional families will be provided in the future.
Note that this key has been developed for mature, i.e. late instar larvae (for which the majority of data are available) and may or may not correctly identify early instars, which can differ substantially from mature larvae.
The first step in using this key is to ensure that your specimen is, in fact, a caterpillar. The vast majority of caterpillars are soft-bodied, grub-like larvae that have prolegs with crochets (hooks) on A3-A6 and A10, and up to six stemmata (simple eyes) on the head. If you are unsure that your specimen is a caterpillar, use the CSIRO Key to Insect Orders to determine the correct insect order.
This key is designed to identify specimens to family only. There are several online resources available that may help in identifying your specimen to genus or lower. For example,
A glossary of entomological terms has been provided.
The key has been designed to expand sequentially, such that characters available first require minimum prior knowledge or expertise. This does not necessarily imply taxonomic importance or familial relatedness. In several cases, a family identification can be successfully accomplished using only a few of these characters. In others, microscopic features will be required for accurate diagnosis. The Fact Sheets, if available for that family, will provide further confirmatory information for confident identification.
Note that figures and line drawings in the key relate only to the relevant character state. As many families have similar identifying features, and it is unrealistic to provide images for every taxa, the image provided may be a different family to that which is finally keyed out. It is for this reason that we have intentionally not labelled character images with taxonomic names.
When using this key, differences from the general larval form in the specimen examined may identify the family quickly, so it is recommended that users familiarise themselves with the gross external anatomy of caterpillars (Figs 1 and 2).
Note that it can be difficult to examine many features in live, active caterpillars, therefore we suggest that specimens being examined should first be killed and preserved (see below). If killing specimens is not suitable, the animal can be immobilised temporarily without harm by placing in a refrigerator (not freezer).
The key opens with only a few features that you must score first to progress with the identification process. This hierarchy of steps usually provides the shortest way to identification as many families can be diagnosed with only a few features. The user can also make use of the feature sets that include unusual or distinctive characters as these may provide a quick diagnosis. We strongly recommend that the key is used in the order that the features are presented (e.g. use General Morphology before progressing to Detailed Morphology.)
Examine your caterpillar first and look for unusual features that stand out; the key is designed to identify families with these characters. It is important to consider all options in a feature set before scoring, as some morphological features may at first appear similar (e.g. crochet arrangements can have very subtle differences). If the user is uncertain about how to score a feature, we recommend that another feature is used instead. Any features that must be scored to progress usually have an ‘unknown’ option.
Ideally, the key should be used to identify whole specimens with no parts missing, including any external structures that the animal has constructed (e.g. larval cases, rolled leaf retreats), and with information on the host. We recognise that sometimes specimens may be damaged, especially at border control, and we have attempted to allow for that.
Generalised features of larval Lepidoptera used in this key include the following:
Note that there are several unusual lepidopteran families that diverge considerably from this basic larval plan. These anomalies are mostly covered in the key.
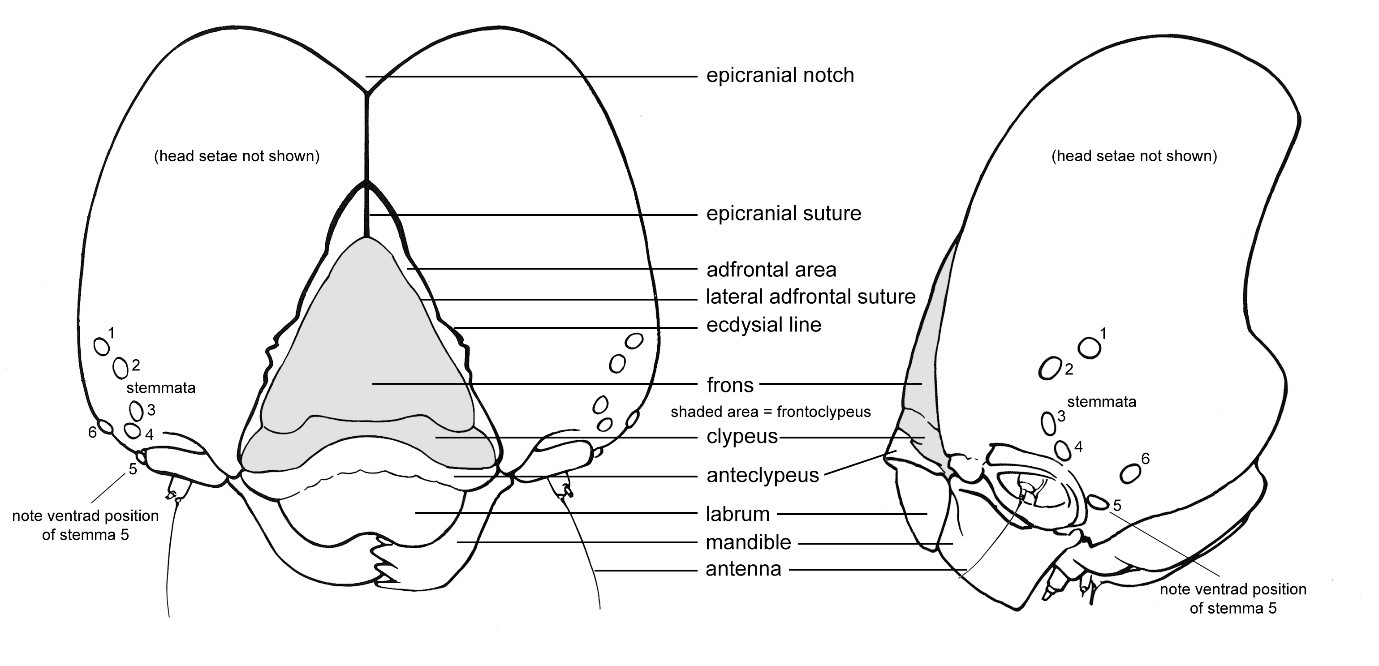
Figure 1. Head capsule of a generalised caterpillar. Note that setae are not shown and are not used in the key. Adapted from Stehr et al. (1987).
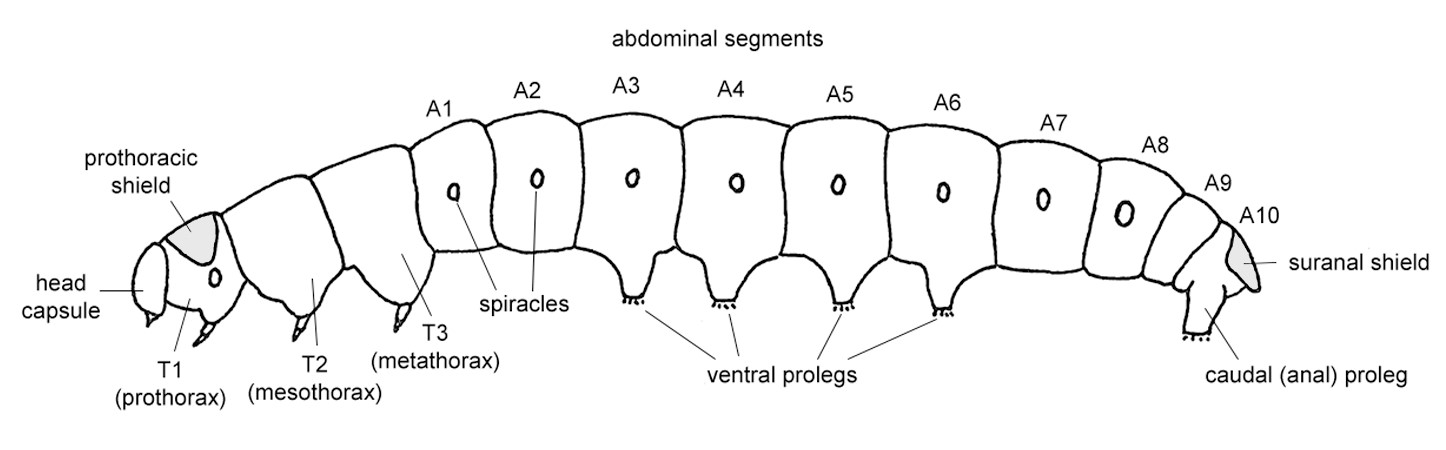
Figure 2. Whole body gross morphology of a generalised caterpillar. Adapted from Carter & Kristensen (1999).
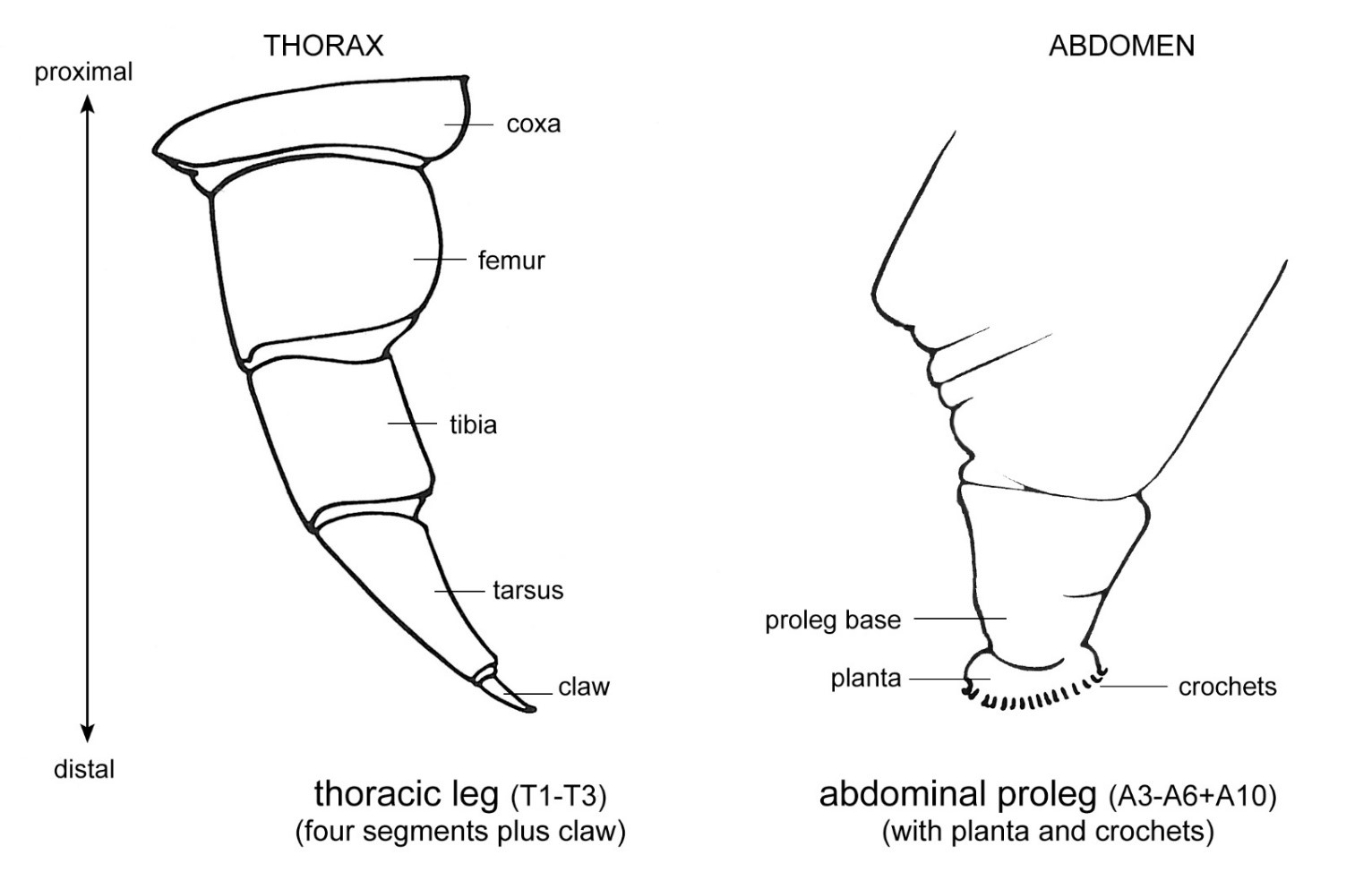
Figure 3. Basic thoracic and abdominal leg types of caterpillars. Adapted from Stehr et al. (1987).
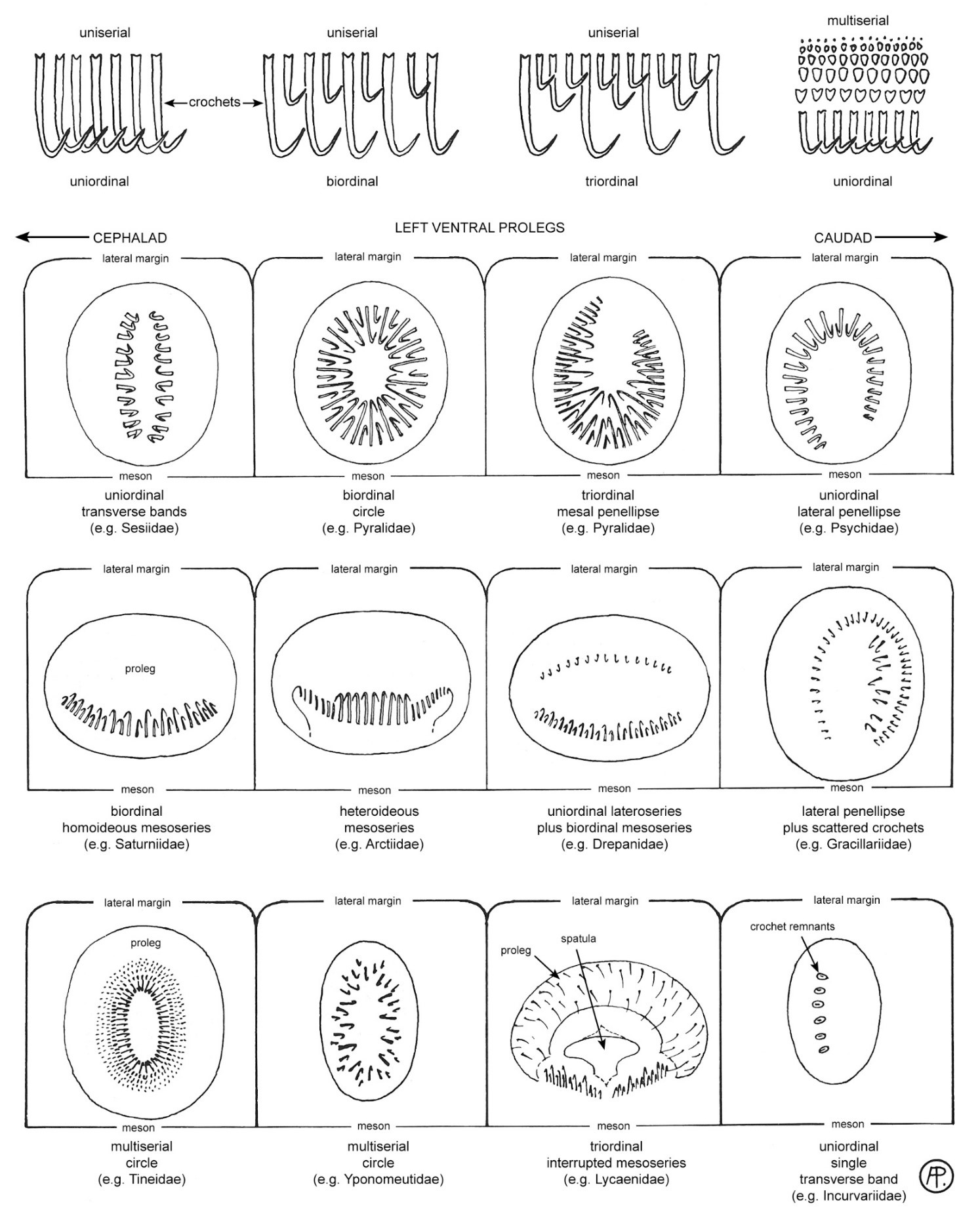
Figure 4. Crochet arrangements and terminology (Peterson 1948) used in the key. Adapted from Stehr et al. (1987).
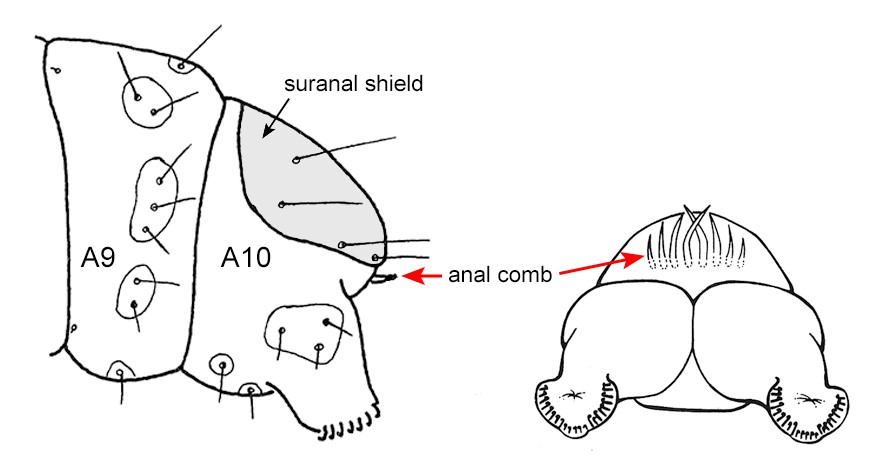
Figure 5. Location and shape of anal comb. Note the shape is variable but is always in the form of a comb, fork or prong. Adapted from Carter & Kristensen (1999) and Stehr et al. (1987).
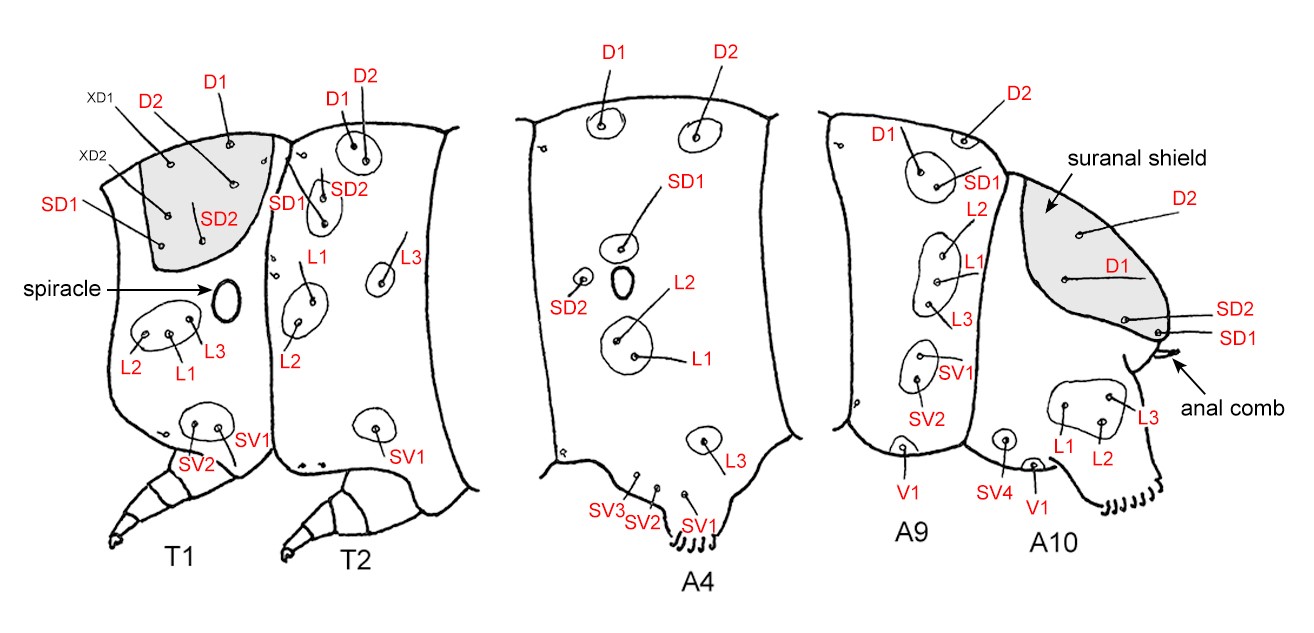
Figure 6. Arrangement of setae used in the key (red). D=dorsal, L=lateral, SD=subdorsal, SV=subventral, V=ventral. Adapted from Carter & Kristensen (1999).
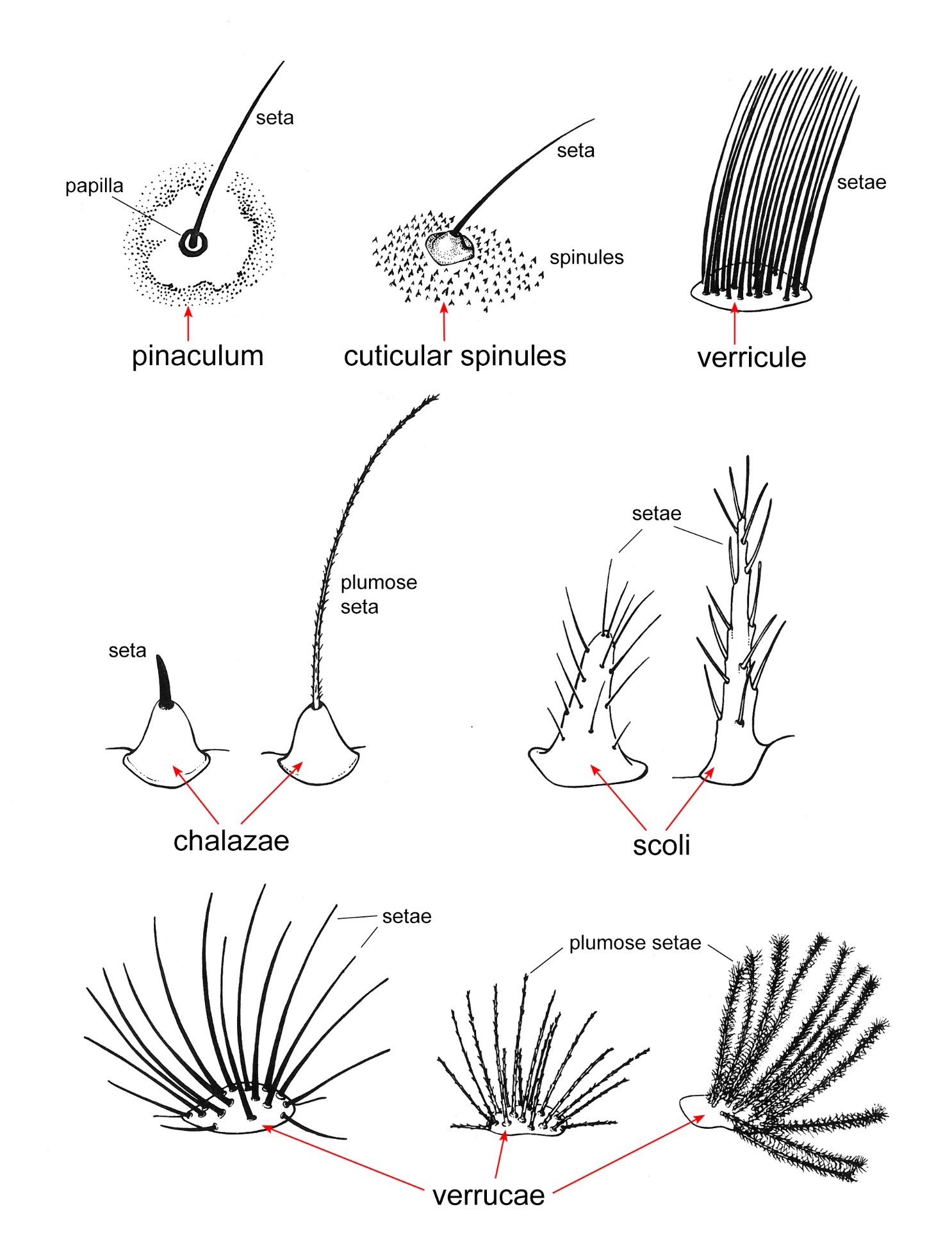
Figure 7. Seta(e)-bearing structures of the caterpillar integument used in the key. Adapted from Stehr et al. (1987).
The number, location and sometimes size of setae (hairs) on a caterpillar’s body are very important in identification and classification, particularly at higher taxonomic levels. Caterpillars grow in size by moulting through a series of stages or instars. Setae that appear in the first instar, and in most cases in the second instar are called primary setae. The position and number of primary setae are highly conserved and can be very useful in diagnosing specimens. Less taxonomically useful at the family level are head capsule setae, so only thoracic and abdominal setae, excluding the miniscule proprioceptor setae, have been utilised in this key. Secondary setae vary widely in number and location and in the majority of cases only appear after the first instar, and are not used in chaetotaxy.
Caterpillar setae are labelled in a standardised way, according to their location on the body, as in Table 1. Note that the number of setae in this table is only a guide; there are exceptions in many families.
Table 1. List of the abbreviations and numbers of the primary setae located in the six main areas on the body of a typical caterpillar.
Carter DJ & NP Kristensen (1999). 3. Classification and Keys to Higher Taxa. In: Kristensen, NP (ed.),
Handbook of Zoology. 4(35.1). De Gruyter, Berlin, New York.
Kristensen, NP (ed.),
Handbook of Zoology. 4(35.1). De Gruyter, Berlin, New York. 1999
Mitter, C, DR Davis & MP Cummings (2017). Phylogeny and Evolution of Lepidoptera. Annu. Rev.
Entomol. 62:265–83
Stehr FW, PJ Martinat, DR Davis, DL Wagner, JB Heppner, ME Brown, ME Toliver, JY Miller, JC Downey, DJ Harvey, N McFarland, HH Neunzig, GL Godfrey, DH Habeck, JE Appleby, M Jeffords, JP Donahue, JW Brown & DC Frack (1987). Order Lepidoptera, pp 288–596. In: Stehr, FW (ed.),
Immature Insects. Kendall/Hunt, Dubuque.
van Nieukerken, EJ, L Kaila, IJ Kitching, NP Kristensen, DC Lees, J Minet (2011) Order Lepidoptera
Linnaeus, 1758. In: Zhang, Z-Q (ed.), Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148: 212-221