|
Chemical analysis of plant tissue is also an important technique in the
diagnosis of nutritional
disorders. In annual crops, tissue analysis is most often used in
‘trouble-shooting’ rather than in the recommendation of fertiliser rates.
However, if the tissue samples are collected early in crop growth and analyses
are completed quickly, a corrective fertiliser application may be possible
within the same season.
The interpretation of tissue analyses is based on established
relationships between crop yield and nutrient concentrations in plant tissue
(Figure 1). Critical concentrations are those which separate the sufficient
(healthy) range from the deficient or toxic range. For practical purposes,
critical concentrations are defined as those concentrations associated with 90%
of maximum yield. Between these concentrations is the range of concentrations
required for healthy growth.
|
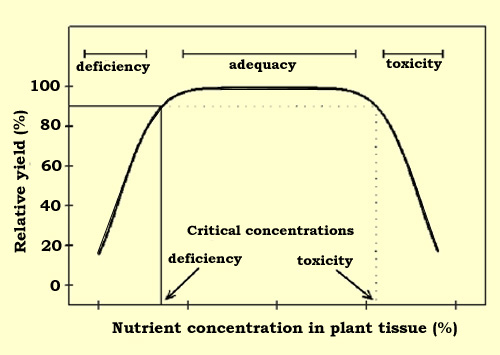
Figure
1. Schematic relationship between relative yield and concentration of a
nutrient in plant tissue. |
The relationship between crop yield and concentration of a
particular nutrient in plant tissue may be determined by means of nutrient
solution culture experiments, glasshouse pot experiments, or field experiments.
Generally, field experiments are considered the best (Bates, 1971), but are
considerably more expensive than solution culture and pot experiments. They also
depend on the availability of sites which are deficient in each of the nutrients
to be studied. The values given in this publication have been derived from
solution culture experiments. Where possible, their reliability has been
validated under field conditions.
A certain part of the plant rather than the whole plant, is
usually collected for analysis. Leaves are usually considered the most
satisfactory parts. Because leaves continue to accumulate some nutrients with
age, it is important that nutrient concentrations in leaves of the same
physiological age are compared. In many annual crops, the blade of the youngest
fully expanded leaf is selected as the ‘index’ tissue for analysis. However,
sweetpotato leaves may continue to expand throughout much of their life, so
their physiological maturity cannot be judged easily on the basis of full
expansion. The many studies referred to in this book have selected different
leaves or parts of the vine for analysis, which makes the information difficult
to compare. For the critical concentrations quoted here, the blades of the 7th
to 9th youngest leaves have been selected as the index tissue. They were
selected as being sufficiently responsive to disorders of both mobile and
immobile nutrients, and having less variable nutrient concentrations than
younger leaves.
|
Critical nutrient concentrations for deficiency and toxicity, and the
adequate concentration ranges for sweetpotato, measured in the 7th to
9th open leaf blades from the shoot tip, sampled at 28 days from
planting. Data were obtained from experiments in solution culture using
cv. Wanmun.
|
|
Nutrient |
Unit |
Critical
Concentration for Deficiency |
Adequate
Range |
Critical
Concentration for Toxicity |
|
N |
% |
4.2 |
4.3 - 5.0 |
|
|
P |
% |
0.22 |
0.26 - 0.45 |
|
|
K |
% |
2.6 |
2.8 - 6.0 |
|
|
Ca |
% |
0.76 |
0.90 - 1.2 |
|
|
Mg |
% |
0.12* |
0.15 - 0.35 |
|
|
S |
% |
0.34 |
0.35 - 0.45 |
|
|
Cl |
% |
- |
- |
0.9 - 1.5 |
|
Fe |
mg/kg |
33 |
45 - 80 |
|
|
B |
mg/kg |
40 |
50 - 200 |
220 - 350 |
|
Mn |
mg/kg |
19 |
26 - 500 |
1600* |
|
Zn |
mg/kg |
11* |
30 - 60 |
70 - 85 |
|
Cu |
mg/kg |
4 - 5 |
5 - 14 |
15.5* |
|
Mo |
mg/kg |
0.2 |
0.5 - 7 |
|
| *These critical concentrations have been
found, on occasion, to be inconsistent with field observations, or to
vary with environmental conditions. Refer to fact sheets on specific
disorders for a full discussion. |
Apart from the physiological age of the leaf, as indicated by
its position on the plant, tissue composition may vary with the age or stage of
development of the crop. For instance, some researchers have found the critical
concentration for N deficiency to decline with crop age. Caution should
therefore be exercised in applying the critical concentrations established for
young plants to tissue taken from older crops. With regard to the values given
in the above table, it should be noted that the sweetpotato plants grew more
rapidly under experimental conditions than could be expected in the field. These
plants may be equivalent to crops from 6 to 10 weeks of age, depending on the
temperature and water supply experienced by the crop. In any case, they
represent crops prior to the stage of rapid growth of the storage roots. From
the point of view of applying corrective measures to the crop on the basis of
the diagnosis, it is preferable to sample the crop at as early a stage as
possible. However, in many instances, symptoms of a disorder may not appear
until advanced stages of crop development. In such cases, tissue analysis will
still be valuable, but discrepancies arising from plant age should be borne in
mind when interpreting the results.
Environmental conditions may further affect the
concentrations of nutrients found in leaves. The concept of critical nutrient
concentration requires that the nutrient of interest is the only factor limiting
growth when the plant material is sampled. It has been shown that water stress
can change the nutrient concentrations in leaves, and that plants take some time
to recover normal concentrations after restoration of adequate water supply. It
has been recommended that a period of several weeks without water stress should
precede the sampling of tissue. This is not practical in many situations, but
users should be mindful of this potential source of error.
To collect leaf samples, the blades are removed without the
petiole, and should be dried as soon as possible after sampling, using gentle
heat (eg. 60-70oC for 48 hours) or microwaving. If samples must be
stored for more than a few hours before drying, it is preferable to keep them
cool (eg. in an ice box, but not in contact with water) to minimise the weight
loss due to respiration of the living tissue. It is important to sample leaves
that have not been contaminated with soil. If the leaves are dusty, they may be
gently rinsed and blotted dry, but extended immersion in water and rubbing
should be avoided. Only distilled or deionised water should be used.
When sampling a crop, it is best to take a composite sample
of leaves from several plants which are equally affected by the symptom of
concern (Reuter and Robinson, 1986). If the crop is not uniformly affected,
several samples could be taken, each from small, uniform areas of the crop, from
the most to the least affected. The symptoms observed, and the level of
severity, should be recorded for each sample, and the samples clearly labelled.
Source: O’Sullivan,
J.N., Asher, C.J. and Blamey, F.P.C. (1997) Nutrient Disorders of Sweet Potato.
ACIAR Monograph No. 48, Australian Centre for International Agricultural
Research, Canberra, 136 p. |
Previous Page:
Diagnosing nutritional
disorders
Next Pages:
Visible symptoms of nutritional
disorders
Soil analysis
Related topics:
Soil
management
Plant
nutrients
Fertilization
Causes
of nutritional disorders
Correcting
nutritional disorders |

