|
Soil structure is
determined by the types of particles that make up the soil, and the way they
stick together. Soil is often described on the basis of the size distribution of
the mineral particles: sand, silt and clay.
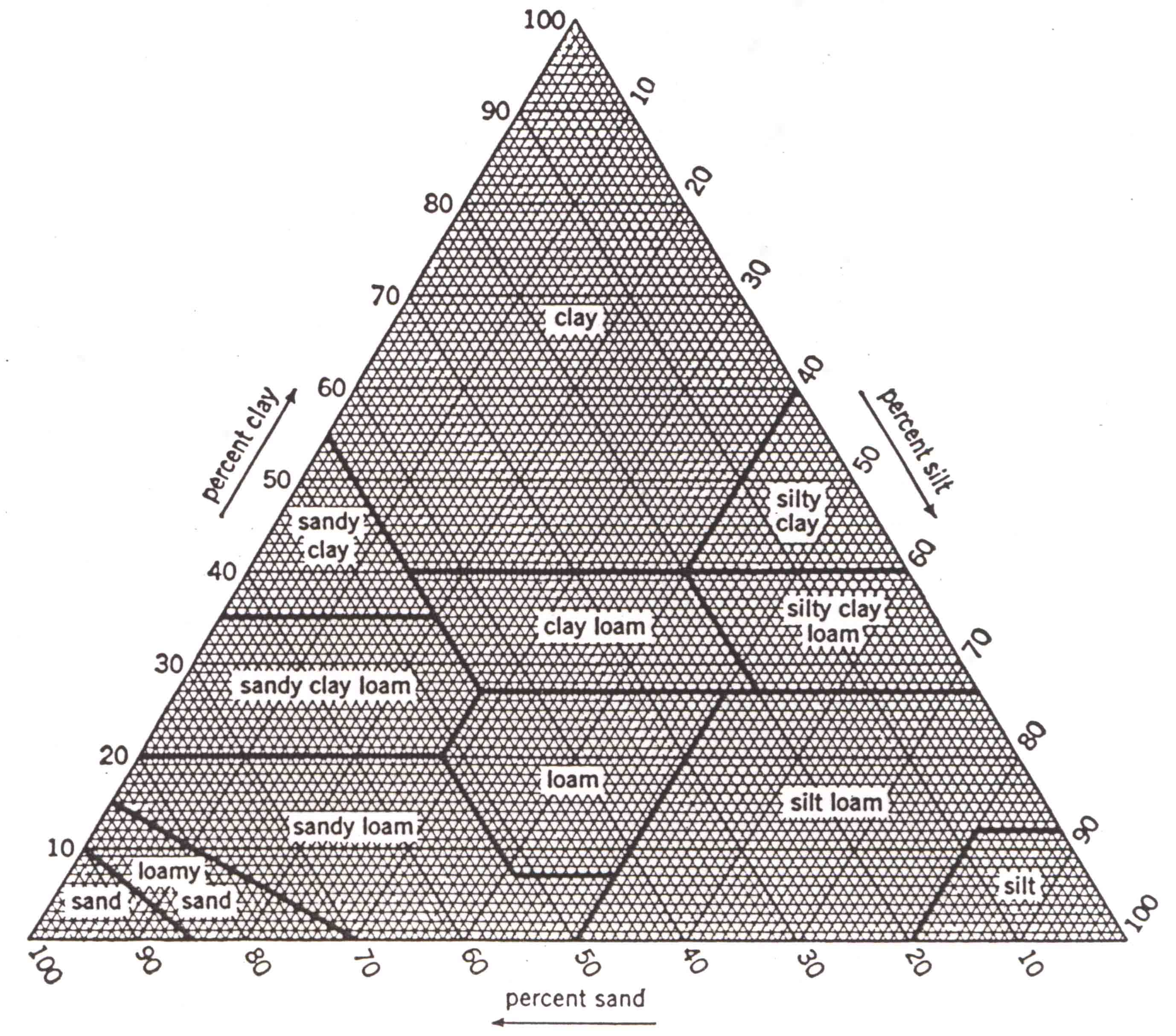
Diagram
of U.S.D.A. soil textural classes
From the plantís perspective, the size of soil particles is less important
than the size of the spaces between them. Relatively large spaces are needed, to
allow water to infiltrate easily, and to drain freely allowing air to re-enter
after wetting. Smaller spaces are also needed to hold water, and to provide
plenty of contact between soil particles and soil water so that nutrients on the
particles can dissolve and become available to plant roots.
Generally, large particles (sand and stones) pack loosely with large spaces
between. Very fine particles (clay) pack very densely with little space. A well
structured soil has small particles clumped together in aggregates, so that
there are both small spaces (between the particles within the aggregates) and
larger spaces (between the aggregates).
If soil aggregates are broken down, for example by plowing when the soil is
too wet, the soil becomes dense and hard for roots to penetrate. Water inside
the heavy clods is barely accessible to roots, and the large spaces around the
clods dry out rapidly.
The stability of soil aggregates is promoted by having appropriate ratios of
Calcium to Potassium and Sodium. If too much Calcium is displaced by Sodium or
Potassium, the bonds between the clay particles are loosened so that aggregates
disperse when wet, and the soil feels heavy and sticky. This can happen over a
period of time as a result of irrigation with slightly saline water (salt
contains sodium), or persistent use of Potassium fertilizer without Calcium
additions. Application of Calcium is needed to restore the soil structure.
Calcium is contained in superphosphate fertilizers, and many farmers have
observed that these fertilizes reduce soil stickiness and improve its
manageability. However, a much cheaper source of Calcium is Gypsum.
Superphosphate should only be applied in amounts needed to meet the cropís
phosphorus requirement, because surplus phosphate can leach into waterways and cause
pollution. Farmers with sticky soil should be encouraged to try gypsum, or lime
if the soil is also acidic.
Soil organic matter is also very effective in stabilizing soil structure.
Microscopic fibres from decomposed plants, as well as the living filaments of
soil fungi, can bind mineral particles together in stable aggregates. Larger
pieces of partially decomposed plant material act as aggregates themselves,
holding water and nutrients inside but allowing free drainage around them. Even
after plant material is completely decomposed, there are organic chemicals, such
as tannins, which decay more slowly, and which can help to bind clay particles
together.
Contributed
by:
Jane O'Sullivan |
Further
topics on Soil Management:
Soil management
Soil
organic matter
Plant
nutrients
Fertilisation
Causes
of nutritional disorders
Diagnosing
nutritional
disorders
Correcting
nutritional
disorders
Other topics on Crop Management: Land
preparation
Production
of planting materials
Planting
Soil
fertility
management
Vine
lifting
Integrated
pest management
Harvesting
Postharvest
practices

Examining the soil profile in a pit dug alongside a sweetpotato field
in PNG (J. O'Sullivan). |

