Background
The Cossidae (cossid millers, goat moths, cossid moths) is a global family of mainly large moths with around 700 named species. The often large caterpillars of this family are usually wood-borers, but sometimes feed under bark, externally on roots and sometimes in the stems of non-woody plants such as cactus. Cossid caterpillars attack a large number of deciduous, non-deciduous and to a lesser extent coniferous plants. Cossid wood-borers burrow deep into trunks and stems, pushing frass and debris to the outside. They pupate in the tunnel, with the pupal shell extruding from the burrow once the moth emerges. The caterpillars of one of the biggest Australian species, Endoxyla leucomochla, are commonly known as witchetty or witjuti grubs. There are almost 100 named species of Cossidae in Australia.
Subfamilies
- Catoptinae
- Chilecomadiinae
- Cossinae
- Cossulinae
- Hypoptinae
- Mehariinae
- Politzariellinae
- Pseudocossinae
- Stygiinae
- Zeuzerinae
Short Description
Medium to very large. When mature, length is approximately 20-150 mm.
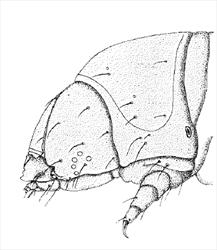
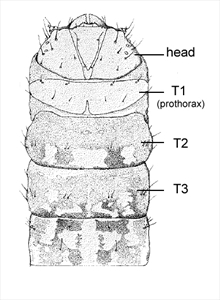
The head is relatively small, and semiprognathous (Fig. 1, Fig. 2, Fig. 3) to hypognathous. The mandibles are enlarged (Fig. 3, Fig. 4). The body is almost cylindrical and usually stout (but can be flattened in the Zeuzerinae), often white, yellow, or pink (Fig. 1, Fig. 2), and sometimes dusky dorsally. The first thoracic segment is often humped and/or rugose (Zeuzerinae) (Fig. 1). On each segment there may be as many as four extra setae present dorso-caudally above the spiracle and sometimes extra SV setae.
Diagnosis
Adapted from Stehr et al. (1987), Common (1990) and Edwards et al. (1999).
Cossid caterpillars are usually wood-borers. The other wood-boring caterpillars that are superficially similar to cossid larvae are sesiid (clearwing moths) larvae and also hepialids (ghost moths) (see below). Cossid larvae are different to sesiids on the following characteristics:
- SD2 is caudad (Fig. 3, Fig. 5) or dorso-caudad of SD1 on T1. In Sesiidae, SD2 is nearly dorsad of SD1 on T1.
- Uni-, bi-, or triordinal crochets in an ellipse, penellipse, or transverse bands. Sesiids only have uniordinal crochets in transverse bands.
- Many cossids have a rugose or humped caudal area on the T1 prothoracic shield (Fig. 1). Instead, sesiids have oblique or sclerotised ridges in the same area.
- Mandibles are enlarged (Fig. 4).
Cossid caterpillars could also be confused with larvae of the Hepialidae (ghost moths). Hepialids can also be large and are commonly wood and stem borers. Wood-boring caterpillars of both can be superficially similar, often being large and stout and with reduced prolegs. However the larvae of Hepialidae are very distinct, mainly on setal characteristics (see Stehr et al. 1987). As in the Cossidae, the prothoracic shield is large and well-developed, but in the hepialids it extends ventrally to include all the lateral setae, or with L3 nearly included; whereas in cossids, the lateral setae are separated from the shield (Fig. 3). On abdominal segments A1-A8, SD2, which is anterior to SD1, is conspicuous, a feature which is unique in Lepidoptera, as on these abdominal segments, SD2 is normally tiny, if not absent, in most other families. Also, crochets in the Hepialidae are uniordinal ellipses, whereas in the Cossidae they can be multiordinal. Another distinguishing feature is that the humped T1, present in in the Zeuzerinae (Fig. 1), is not a feature of hepialids.
Detailed Description
Adapted from Stehr et al. (1987) and Edwards et al. (1999).
(Fig. 1, Fig. 2, Fig. 3)
The body is rather stout and cylindrical, but may be flattened in the Zeuzerinae.
Head: Semiprognathous to hypognathous, relatively small and often wedge-shaped. The frontoclypeus usually extends one-half or less the distance to the epicranial notch. An exception is Coryphodema tristis (South African cossid moth) (Cossinae) in which the frontoclypeus extends more than halfway to the notch (Fig. 3, Fig. 6). The ecdysial lines are usually bowed slightly inward near the centre and usually meet about halfway between the apex of the frons and the epicranial notch (not in C. tristis). There are six stemmata, which are usually evenly spaced in a semicircle except sometimes for number five, which is ventral to the antenna (Fig. 3).
Thorax: The prothoracic shield is large and well-sclerotised, but does not extend to include the lateral setae (Fig. 3), and the T1 dorsum is frequently humped and rugose or spined (in the Zeuzerinae). SD2 is nearly caudad to SD1 on T1 (Fig. 5). The L group is trisetose on T1 to T3 (Fig. 5). One exception to this last state is the Central and South American genus stemborer, Langsdorfia, in which the prespiracular L group is quadrisetose on T1. This is unique, or at least extremely uncommon, in the Lepidoptera, as far as we know. L1 is usually on a separate pinaculum and closer to L3 than L2 on T2 and T3 (not in C. tristis), the SV group is bisetose on T1 (Fig. 5) and unisetose on T2 and T3 (Fig. 5) (rarely bisetose).
Abdomen: The D2s are usually further apart than the D1s on A1-7, often closer together on A8 and A9 and are sometimes closer together on the more anterior segments. SD1 is large and dorsal to the spiracle on A1-7 (Fig. 5), SD2 is small to minute and anterior of the spiracle on A1-7 (Fig. 5). SD1 and SD2 positions are more variable on A8. L1 and L2 are on the same pinaculum ventral to the spiracle on A1-8 (Fig. 5). L3 is closer to the SV group on A1-8 than to the L1 and L2 group. The SV group is usually bisetose on A1 and A8, and trisetose on A2-7 (Fig. 5). All the A9 setae are usually in a vertical line except D1, which is anterior. The A10 shield bears four setae per side. The A10 crochets are sometimes uniordinal.
The prolegs on A3-6 and A10 are usually stout and short or vestigial. The crochets are irregularly uni-, bi-, or triordinal and are arranged in an ellipse, or more rarely in a penellipse, or transverse band(s). The spiracles are often large and round to oval on T1, A8 spiracles are sometimes two or three times larger than the A1-7 spiracles, and the A8 spiracle sometimes projects posteriorly and is closer to the midline than the others.
Species of Biosecurity Concern
THE FOLLOWING SPECIES ARE OF BIOSECURITY CONCERN TO NORTHERN AUSTRALIA
Polyphagozerra reticulata (red borer) (Zeuzerinae)
Polyphagozerra coffeae (red coffee borer, coffee carpenter) (Zeuzerinae)
The main cossid moth of biosecurity concern to northern Australia is red borer (Polyphagozerra reticulata) (Zeuzerinae). There are two species in the genus. The other is red coffee borer or coffee carpenter (Polyphagozerra coffeae). P. reticulata is a pest in Papua New Guinea (PNG) and the Moluccas in mainly coffee plantations, but also attacks other plants (see below). The similar P. coffeae is considered to be a more serious pest in coffee plantations and other crops but does not occur in PNG. It is widely distributed in Asia and is notorious for being the most polyphagous species of the Cossidae with its association with plants of 34 families (Yakovlev 2012) (see below).
The erection of the new genus Polyphagozerra for these two species by Yakovlev (2011) remains controversial and some workers still use the previous generic placement under Zeuzera.
Description
No information on the morphology of Polyphagozerra reticulata can be found to date. The following is a description of the superficial features of the mature caterpillar of its congener, Polyphagozerra coffeae:
Head semi-prognathous, mainly golden yellow to brown, tinged with pink. Mouthparts are dark brown.
Body mainly dark pink, becoming much lighter in the thoracic region. Length is approximately 40 mm.
Thorax – Dorsum of T1 and T2 light yellow, T3 dorsum dark pink. T1 prothoracic shield very well-developed, golden to dark brown, well-sclerotised and divided centrally, T1 with a dorsal, humped, rugose, folded section caudally. Legs short, light brown with claws.
Abdomen – dark pink, with setae on slightly domed, large, darker pink pinacula. With a narrow yellow stripe on the mid-lateral line. The dorsum of the A8 segment, pale yellow on the posterior half. Dorsum of A9 with a very dark brown mid-dorsal sclerite. The A10 dorsal suranal shield, very dark brown, well-sclerotised, and rounded posteriorly. The anal claspers have a dark brown sclerite just ventral of the lateral line. Prolegs on A3-6 are moderately short.
Setae – moderately long, acute, white. Spiracles – Yellow and oval. Spiracles on T1 and A8 are relatively large and almost twice as long as the others on the abdominal segments.
Biology and Feeding Damage
Little information has been published on the biology of Polyphagozerra reticulata. The following details relate to P. coffeae, which is likely to have a similar biology to its congener.
- The female lays eggs, arranged in rows, in cracks on the stems or branches of host plants (see below).
- The caterpillars hatch in around ten days.
- Larvae bore into stems and branches to feed on wood.
- Hibernation occurs in the larval stage from November to January (Ahmad 2017).
- Feeding has been reported as beginning in February in Pakistan (Ahmad 2017).
- Larvae cut an opening in the outer wall of the tunnel before pupating (Ahmad 2017).
- Pupation extends for 17-21 days (Ahmad 2017).
- Stems show the effects of dieback because of internal feeding, wilt, become brittle and often break.
- Frass is exuded from the entrance holes and piles of frass are often evident at the base of the plants.
Current Distribution
Polyphagozerra reticulata
- Papua New Guinea
- The Moluccas
Polyphagozerra coffeae
- Asia
Caterpillar Host Plants
Polyphagozerra reticulata
- Coffea (coffee)
- Camellia sinensis (tea)
- Gossypium (cotton)
- Theobroma cacao (cocoa)
- Ceiba pentandra (kapok)
- Erythroxylum (coca)
- Tectona grandis (teak)
Polyphagozerra coffeae
- Casuarina
- Erythroxylum (coca)
- Acalypha, (copperleaf, three-seeded mercury)
- Phyllanthus
- Hydnocarpus
- Annona (including paw paw)
- Cinnamomum (including cinnamon)
- Persea (including avocado)
- Phoebe
- Amherstia (Pride of Burma)
- Cassia (cassia)
- Pericopsis
- Xylia
- Gossypium (cotton)
- Hibiscus (hibiscus)
- Cedrela
- Chukrasia (Indian mahogany)
- Melia
- Swietenia (mahogany)
- Psidium (including guava)
- Grevillea (grevillea, spider flower, silky oak and toothbrush plant)
- Crataegus (hawthorn, quickthorn, thornapple, May-tree, whitethorn, or hawberry)
- Eriobotrya
- Coffea (coffee)
- Citrus (citrus)
- Santalum (including sandalwood)
- Filicium
- Nephelium (including rambutan)
- Schleichera oleosa (kusum tree, Ceylon oak, lac tree, gum lac tree)
- Cestrum (cestrums, jessamines)
- Theobroma (including cocoa)
- Cryptomeria japonica (Japanese sugi pine, Japanese red-cedar)
- Camellia (including tea and camellias)
- Clerodendrum (glorybower, bagflower, bleeding-heart)
- Lantana (shrub verbenas or lantanas)
- Tectona (teak)
- Vitex (chastetree)
(CABI 2019)
***
Coryphodema tristis (South African cossid moth) (Cossinae)
The South African cossid moth, Coryphodema tristis, is a native species of South Africa and is well known as a pest of fruit trees and vines as well as native trees in that country (Hoppner & Ferriera 1990). Additionally, caterpillars of this species are wood borers and cause serious damage to Eucalyptus nitens plantations in South Africa. This moth represents a serious risk to Australia's forest industry and is listed as a potential high priority plant pest by the Department of Agriculture.
The South African cossid moth was described comprehensively, diagnosed and a detailed prognosis of its biosecurity status and identification provided by Young (nee Byrne) (2010).
Description
(Fig. 2)
Length (from Pettey 1917): 32-38 mm
Length of mature caterpillars feeding on Eucalyptus nitens: range 37-43 mm; 40.75 ± 1.44 (SE) mm (n=4). This difference in size of the larvae possibly reflects the branch size of the host as proposed by Pettey (1917), as Pettey’s specimens originated from fruit trees.
Head (Fig. 4): The head capsule is strongly rugose, with the dorsum dark reddish brown and the venter pale off-white. The antennal basal segments are pale pinkish-brown and the apical segments dark reddish-brown. The clypeus is pale brown, the labrum dark brown and the mandibles dark reddish-brown. The spinneret is short. There are six, pale yellow stemmata. Chaetotaxy is illustrated in (Fig. 3).
Body (Fig. 2): The ground colour pale on the dorsal surface is brownish-yellow with numerous maroon blotches and several wavy maroon stripes. The prothoracic shield is strongly sclerotised with a mid-dorsal transverse brown stripe broken mid-dorsally. The ground colour of the venter is somewhat paler than the dorsum and marbled with pale maroon, except on A3-A6, which are plain pale yellow. There is a moderately flattened, crenulated, fleshy, longitudinal protuberance just ventrad of the lateral line, flecked with pale maroon blotches. Thoracic legs are light brown, but paler on the inner side and with dark brown claws. Spiracles are oval with dark brown rims, and a pale yellow centre with a reddish-brown central opening. The suranal shield is rugose, and centred by a dark-brown sclerite. Setae are brown and moderately long but shorter on the venter, and acuminate. Papilla are small, slightly raised and yellowish-brown.
Prolegs are very short and stout, and are present on A3-A6, A10. Crochets on A3-A6 are triordinal, uniserial, and arranged in transverse ellipses (approximately 90 per proleg). On A10 crochets are biordinal, uniserial, and arranged in a transverse band (approximately 27 per proleg).
Chaetotaxy is illustrated in Fig. 5.
Biology and Feeding Damage
Immature Larvae: First instar larvae feed under a protective cover consisting of a thin layer of silk and frass. Hatching occurs in December and January. After a few days the young caterpillars burrow into the bark and feed for at least two months on the cambium. During this period they continue to produce silk webbing combined with frass, which conspicuously marks the larval feeding location. If webbing is removed, larvae immediately escape into the feeding burrows (Pettey 1917).
Mature Larvae: After about eight weeks from hatching, from late summer to early autumn, the larvae bore deeper into the sapwood and heartwood of the tree (Fig. 7). The larvae then actively feed for approximately eighteen months. In quince trees, larvae appear to disperse into smaller feeding groups over time. Burrows are not discrete but are joined together (Fig. 9). Openings are formed at intervals along branches or the trunk and where frass is pushed to the outside of the tree (Fig. 8). Larvae of one generation are active for around 18 months. Mature larvae can usually be found during most of the year except late winter to early spring (Pettey 1917).
Branches and trunks of young to mature live trees are attacked by this species and the following damage occurs in Eucalyptus nitens:
- Early, shallow feeding damage by young larvae is marked by a protective layer of silk webbing and frass.
- Mature larval damage:
- Round holes penetrating the sapwood (Fig. 9)
- Resin (Fig. 10) and frass resembling ‘sawdust’ appearing on trunks and branches (Fig. 8)
- Extensive tunnelling of larvae in sapwood and heartwood (Fig. 7, Fig. 11)
- Pupal cases protruding from emergence holes or on the forest floor (Fig. 12)
- Frass resembling ‘sawdust’ at the base of trees (Fig. 13)
- Unpleasant odour
- Darkening of bark in infested trees (only observed in E. nitens) (Fig. 14).
Current Distribution
Botswana and South Africa
de Prins & de Prins (2017)
References
Ahmad, I. (2017). Integrated pest management of Zeuzera coffeae Nietner: an efficient approach to reduce the infestation of walnut trees. Pakistan J. Zool., 49(2): 693-698, 2017. DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.2.693.698
Centre for Agriculture and Bioscience International (CABI) (2019). Plantwise Knowledge Bank. Zeuzera coffeae (coffee carpenter). https://www.plantwise.org/knowledgebank/datasheet/57492. Accessed September 2019.
Common, I. F. B. (1990) Moths of Australia, E.J. Brill and Melbourne University Press. Melbourne.
de Prins, J. & de Prins, W. (2017). Coryphodema tristis (Drury, 1782). Afromoths. Retrieved September, 2019. http://www.afromoths.net/species/show/7549
de Vos, R. The Goat Moths (Lepidoptera: Cossidae) of Papua Indonesia. https://www.papua-insects.nl/insect%20orders/Lepidoptera/Cossidae/Zeuzera/Zeuzera%20reticulata.htm
Edwards, E. D., Gentiii, P., Horak, M., Kristensen, N. P., & Nielsen, E. S. (1999). The Cossoid/Sesioid Assemblage. In: Kristensen, N.P. (Ed.), Handbook of Zoology. 4(35.1). De Gruyter, Berlin, New York.
Hoppner, G. F. J. & Ferriera, J. H. S. (1990). Fungi associated with the quince borer, Coryphodema tristis (Drury) (Lepidoptera: Cossidae), in grapevines. South African Journal of Enology and Viticulture. 11(2): 67-69.
Pettey, F. W. (1917). The quince borer and its control. Department of Agriculture Report, Union of South Africa. No. 2.
Stehr, F.W., Martinat, P.J., Davis, D.R., Wagner, D.L., Heppner, J.B., Brown, M.E., Toliver, M.E., Miller, J.Y., Downey, J.C., Harvey, D.J., McFarland, N., Neunzig, H.H., Godfrey, G.L., Habeck, D.H., Appleby, J.E., Jeffords, M., Donahue, J.P., Brown, J.W. & Frack, D.C. (1987) Order Lepidoptera, pp 288–596. In Stehr, F. W. (Ed.), Immature Insects. Kendall/Hunt, Dubuque.
Yakovlev, R.V., 2011: Catalogue of the Family Cossidae of the Old World. Neue Entomologische Nachrichten, 66: 1-129.
Yakovlev, R.V. (2012) Trophic relations of Old World carpenter-moths (Lepidoptera, Cossidae). Evraziatskii entomologicheskii zhurnal, 11(2): 189-194.
Young (nee Byrne), C. J. (2010). South African Cossid Moth. Diagnostic Scholarship Report. Tasmanian Department of Economic Development, Tourism and the Arts.
 Coryphodema tristis
Coryphodema tristis