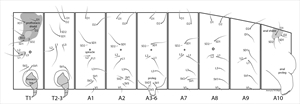
Background
The micromoth family Gelechiidae is the namesake family for the enormous superfamily Gelechioidea and is one of the most diverse families of the Lepidoptera. Gelechiids are very small moths with narrow wings. The family has a worldwide distribution and is diverse with over 4,700 known species in 500 genera, but that number would be at least doubled with undescribed and new species. The family is particularly prevalent in North America because of the close association of species with Douglas fir (Pseudotsuga).
The caterpillars have very diverse feeding habits on many plant parts and species. Many species are external feeders but are protected by a shelter of some kind, often in tied or rolled leaves (Fig. 1). Many others feed internally, boring into flower heads, stems (Fig. 2) or roots. They are leaf-miners (Fig. 11), gall-inducers or stem-borers, or feed within flowers or fruits (Fig. 13). They also can be concealed in silken tunnels in earth or in portable cases. Not surprisingly, therefore, there are many gelechiids that are agricultural pests, such as: the pink bollworm, Pectinophora gossypiella (Fig. 13), one of the most destructive pests of cotton in the world; Sitotroga cerealella (Angoumois grain moth); Phthorimaea operculella (potato tuber moth); and, Tuta absoluta (tomato leaf miner). Contrastingly, several species have been used as biocontrol agents against weeds. One Australian species feeds on dead leaf litter.
Subfamilies
The Gelechiidae consists of the following seven subfamilies (Karsholt et al. 2013):
- Anacampsinae
- Anomologinae
- Apatetrinae
- Dichomeridinae (formerly including Chelariinae, which is now placed in Anacampsinae)
- Gelechiinae
- Physoptilinae
- Thiotrichinae
Short Description
Adapted from Stehr et al. (1987) and Hodges (1999).
Mature larvae are small to medium. The normal complement of primary setae is present but are sometimes reduced and difficult to see in small leaf miners, and seed or gall inhabitants. Secondary setae are usually absent, but may be present on the A3-A6 prolegs and/or A10 in a few species such as the European pest species, the peach twig borer, Anarsia lineatella (Fig. 2). The integument is usually smooth, but is sometimes granulated. Spiracles are circular, and usually small and distinct with the T1 and A8 spiracles usually larger.
Diagnosis
(Capps 1946, Stehr et al. 1987, Passoa 1995)
Gelechiid larvae can be diagnosed on the following combination of setal characters:
- T1 - trisetose prespiracular lateral setae (Fig. 3, Fig. 16).
- A3-A6 - L1 and L2 closely spaced, below the spiracle (Fig. 16).
- A1 - most gelechiids have 2 SV setae on A1, rarely 1 or 3, whereas most micromoths have 3 SV setae on A1 (Fig. 16).
- A3—A6 – trisetose SV (Fig. 16).
- A9 – D and SD setae each on separate pinacula.
The following characteristic features are also found in many gelechiids:
- Hairlike SD1 on A9 (tonosensillum) – also in the other gelechioid families, Blastobasidae and Oecophoridae (rarely in Cosmopterigidae).
- Anal fork in some species – not present in other gelechioid families. Present in Tortricidae, but tortricids do not have a tonosensillum at SD1 on A9.
- A mandible with an outer (extra) tooth.
- Crochets divided into two groups.
- A sclerotised collar on the abdominal prolegs.
Detailed Description
(Stehr et al. 1987, Hodges 1999)
Head: The head is semi-hypognathous (Fig. 3, Fig. 6, Fig. 13), to prognathous (Fig. 6, Fig. 7) in leaf miners, and usually heavily pigmented and smooth (Fig. 1, Fig. 3, Fig. 4, Fig. 6, Fig. 13, Fig. 14). The frontoclypeus is longer than wide, and usually extending one half to four-fifths to the epicranial notch (Fig. 4, Fig. 15), but all the way in some prognathous species (Fig. 10). The ecdysial lines are usually distinct, close to adfrontal sutures anteriorly, but angling outward posteriorly and usually meeting at the epicranial notch (Fig. 4, Fig. 14). Six stemmata are arranged in a normal arc, with no. 6 being caudad (Fig. 3). Head seta L1 usually further from head seta A3 than A3 is from head seta A2. The integument may be spinulose (Fig. 9).
Thorax: The prothoracic shield is variably pigmented and distinct (Fig. 3, Fig. 4, Fig. 6, Fig. 7, Fig. 10, Fig. 13, Fig. 14). The standard number of primary setae is present. The prespiracular L group on T1 is trisetose, with the middle seta usually distinctly lower (Fig. 3, Fig. 16). The SV group has two setae on T1, and one on T2 and T3. T2 and T3 with setae normally arranged and with L1 closer to L2 than L3 (Fig. 16).
Abdomen: D1 and D2 are wide of the midline, with D2s usually further away. SD1 on A1-A7 is dorsad (Fig. 16) to anterodorsad of the spiracle. L1 and L2 are below the spiracle and close together and L3 is more or less above the caudal margin of the proleg. The SV group is almost invariably bisetose on A1, trisetose on A2-A6, bisetose on A7, and usually unisetose on A8 (rarely bisetose) (Fig. 16). SD1 on A8 is usually dorsad (Fig. 16) or anterodorsad to the spiracle (rarely anterior). A9 has D1 below and usually lined up with D2 and SD1 (Fig. 16), which may be hairlike (tonosensillum); L1 and L2 are close together on the same pinaculum, with L3 close to, distant or absent to the other L setae. SV setae are rarely absent (Fig. 16). A3-A6 prolegs are short (Fig. 6) to 2X as long as the width of the planta at the crochet level. The crochets are arranged in a uni-, partially biordinal circle (Fig. 5) or penellipse (Fig. 15), and may be, rarely, reduced in number or to small lumps (as in the Australian pest species Sitotroga cerealella (Angoumois grain moth)), or almost to 2 transverse rows (e.g. Exoteleia (Holarctic)) or vestigial, or absent (e.g. the Holarctic genera, Metzneria and Isophrictus). Prolegs sometimes each have a sclerotised collar and/or with a sclerotised, central foot patch.
A10 usually has the standard complement of setae (Fig. 16) with secondary setae present in a few species, such as the peach twig borer, Anarsia lineatella (Fig. 2). Prolegs are normal, with transverse rows of uni- or biordinal crochets (Fig. 17) that may be divided by a gap, but are rarely reduced or absent. An anal fork is sometimes present.
Species of Biosecurity Concern
THE FOLLOWING SPECIES OF GELECHIIDAE ARE CONSIDERED TO BE OF BIOSECURITY CONRCERN TO NORTHERN AUSTRALIA
Anarsia lineatella (peach twig borer) (Anacampsinae: Chelariini)
Anarsia is a very large genus of around 100 species that is widely distributed in Eurasia and Africa (Gregerson & Karsholt 2017).
Diagnosis
-
Secondary setae are present on the prolegs, where they are usually entirely absent in other gelechiids.
-
A dark brown anal comb with forked medial prongs is present (anal combs are not present in other gelechioid families).
Description
(Fig. 2)
The head, prothoracic shield & suranal shield are a glistening dark brown to black (fades to a lighter brown in preserved specimens). The prothoracic spiracle shares the same pinaculum as the prespiracular lateral setae (Fig. 3). The legs are black (faded in preserved specimens). The body is reddish-brown, with whitish intersegmental sections, giving a striped appearance. Pinacula are small & dark-brown, setae are white (Fig. 3, Fig. 4, Fig. 5). Peritremes are also black. A dark brown anal comb with forked medial prongs is present. The prolegs are the same colour as the body (Gregersen & Karsholt 2017). The crochets are arranged in a partially biordinal circle (Fig. 5).
Biology and Feeding Damage
This species is multi-voltine, with at least three generations and four in warmer climates. First–generation caterpillars overwinter in small silk-covered cells on the bark of fruit trees. In spring they begin boring into buds and young shoots (Fig. 2) and then move on to fruit after pits begin to harden. Second-generation larvae feed on fruit, causing most crop damage. Third-generation larvae generally inflict little economic damage. Caterpillars pupate in a light web on the ground or between leaves (Gregersen & Karsholt 2017).
Caterpillars feed internally on fruit, stems and flowers. Damage caused by feeding includes the following:
-
gummosis
-
leaf wilting
-
stem dieback
-
stem wilting
Overwintering, first generation larvae bore into young shoots in the spring (Fig. 2). The tips of these shoots then wilt and blacken, particularly in peach (Prunus persica). Second generation larvae destroy fruit, with infested almonds producing a large mass of gum. The entrance hole in fruit is inconspicuous, but the fruit becomes discoloured and matures too early (MAF Plant Health & Environment Laboratory 2011) (Gregersen & Karsholt 2017). For more information see: https://www.cabi.org/isc/datasheet/5154.
Current Distribution
This species is widely distributed and ubiquitous. It has previously been introduced to Queensland and Victoria, but has been successfully eradicated (CABI 2020).
-
Europe
-
North America
-
Africa
-
Asia
For more information on distribution see: https://www.cabi.org/isc/datasheet/5154
Caterpillar host plants
-
Prunus, particularly almonds (P. dulcis), peach (P. persica) and apricot (P. armenica)
-
apple (Malus)
-
quince (Cydonia oblonga)
For more detailed information see: https://www.plantwise.org/knowledgebank/datasheet/5154
***
Brachmia blandella (gorse crest) (Dichomeridinae)
Very little is known about the biology and life cycle of this European species.
Biology and Feeding Damage
This species is univoltine; the immature caterpillar overwinters in a silk web covering on the host plant. Pupation takes place in early summer.
Current Distribution
-
Europe (except Ireland, Slovenia and Croatia)
Caterpillar Host Plants
-
gorse (Ulex europaeus)
***
Keiferia lycopersicella (tomato pinworm) (Gelechiinae: Gnorimoschemini)
This species is a severe pest of tomatoes in the Americas, with economic losses costed at millions of dollars (US) in the USA and Mexico (Trumble 2013).
Diagnosis
-
Pale prothoracic shield with a dark posterior band (Fig. 6, Fig. 7).
-
Abdomen striped with pink or purple bands and stripes (Fig. 6, Fig. 7).
-
Posterior abdominal integument with microspinules (Fig. 8, Fig. 9).
Description
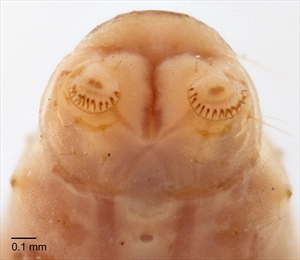
Caterpillars of this species are variable in colour (Fig. 6, Fig. 7, Fig. 8) (Passoa & Young, in Hayden et al. 2013). However, the prothoracic shield is characteristically pale with a dark posterior band, but this may vary among specimens (Fig. 6, Fig. 7, Fig. 8). The head is light to dark brown and prognathous (Fig. 6, Fig. 7), which is typical of gelechiid leafminers. The frontoclypeus is longer than wide, and extends all the way to the epicranial notch, a condition typical of prognathous leafminers (Fig. 10). Crochets are arranged in a penellipse. SD1 on A9 is hair-like (tonosensillum). The abdomen is usually striped with bands or patches of pink or purple (Fig. 7) or sometimes is a more uniform purple (Fig. 8). The integument on the dorsum of the posterior abdominal segments is spinose with blunt or pointed microgranules apparent under high magnification (Fig. 8, Fig. 9) (Hayden et al. 2013). Mature larvae reach a length of 5.8-7.9 mm (Trumble 2013).
Biology and Feeding Damage
This species is multivoltine with seven to eight generations per year. Caterpillars feed exclusively on species of Solanaceae (nightshades), with tomato plants the preferred host. Eggs are laid singly or in small groups on the underside of leaves of the hostplant, but may be laid anywhere on the plant at high densities of the moth. Fresh eggs are pale yellow and become orange before larvae hatch. Larvae develop through four instars in around 9 to 17 days. Immature larvae shelter under a silk covering from which they tunnel into the leaves of the host plant. Mature larvae progress to tied or folded leaves for shelters. Larvae pupate in a loose cell of soil particles near the surface of the soil. Caterpillars also burrow into stems and fruit (Poe 1999, Trumble 2013). In fruit, the entry hole is visible with galleries of burrows and silk obvious just beneath the surface (CABI 2020c).
Feeding in leaves results in a blotch-like mine (Fig. 11). Initially leaf mining damage is slight with most damage caused by larvae feeding on leaves, boring into stems and fruit and leaf tying and folding. Secondary damage is caused by infections of pathogens in the damaged tissues (Poe 1999).
Current Distribution
North America
-
Mexico
-
California
-
Texas
-
Georgia
-
Florida
-
Hawaii
-
Cuba
-
Haiti
-
Bahamas
and as a greenhouse pest in:
-
Delaware
-
Mississippi
-
Missouri
-
Pennsylvania
-
Virginia
Poe (1999). http://entnemdept.ufl.edu/creatures/veg/tomato/tomato_pinworm.htm
Central America
-
Costa Rica
-
El Salvador
-
Guatemala
-
Honduras
-
Nicaragua
-
Panama
South America
-
Bolivia
-
Colombia
-
Guyana
-
Peru
-
Venezuela
Trumble (2013)
This species was introduced into Italy in 2008 but was eradicated (Huemer & Karsholt 2010).
Caterpillar host plants
Species of Solanaceae including:
-
tomato (Lycopersicon esculentum)
-
eggplant (Solanum melongena)
-
potato (Solanum tuberosum)
-
Carolina horsenettle (Solanum carolinense)
-
chaparral nightshade (Solanum xanthii)
-
bluewitch nightshade (Solanum umbelliferum)
-
Bahama nightshade (Solanum bahamense)
-
Other species of Solanum
Poe (1999).
***
Pectinophora gossypiella (pink bollworm) (Apatetrinae: Pexicopiini)
This species is a global, serious pest of cotton (CABI 2020d). In the USA it is commonly intercepted on okra (Abelmoschus esculentus) from the Caribbean.
Diagnosis
This species can be diagnosed on the following combination of characters:
-
Head chaetotaxy
-
Mandible with four teeth, the last one smaller than the others (Fig. 12)
-
Thorax
-
Prothoracic shield crescent shaped (Fig. 13, Fig. 14)
-
-
Abdomen
-
Abdominal prolegs with crochets in a uniordinal penellipse (Fig. 15)
-
A8 - SD1 is dorsad to the spiracle(Fig. 16)
-
A9 - SD1 is setiform, not hairlike (as for a tonosensillum) (Fig. 16)
-
A9 – L group normally bisetose (Fig. 16)
-
A10 - crochets in a single uninterrupted band, anal comb absent (Fig. 17)
-
(Gilligan & Passoa 2014)
Description
Mature caterpillars are pale with prominent pink dorsal bands (Fig. 13). Length of the mature larvae ranges from 12-15 mm.
Biology and Feeding Damage
Eggs are laid by the moths on or near the cotton bolls at the time of flowering. Young larvae immediately burrow into cotton buds after hatching. They feed internally on the seed pod and cut a small hole for air. Larvae develop through four instars. On maturity they emerge from the top of the boll and then burrow into the substrate to pupate about 50 mm from the surface. There are around four to six generations of the species annually. Larvae are known to diapause in a small cocoon in partially opened bolls, stored seed or in the soil under cool and dry conditions. Under these conditions they can remain quiescent for up to 2.5 years (CABI 2020d).
Infestation by this species causes a failure of buds to open, fruit shedding and seed loss (CAB) 2020d).
Current Distribution
Pink bollworm is native to Asia.
Introduced to:
-
Tropical America
-
Africa
-
Australasia (NT, Q, SA and WA)
-
Pakistan
-
Egypt
-
Mexico.
For more detailed information on distribution see: https://www.cabi.org/isc/datasheet/39417.
Caterpillar Host Plants
-
okra (Abelmoschus esculentus)
-
Indian mallow (Abutilon)
-
country mallow (Abutilon indicum)
-
hollyhocks (Althaea)
-
cotton (Gossypium)
-
tree cotton (Gossypium arboreum)
-
short staple cotton (Gossypium herbaceum)
-
rosemallows (Hibiscus)
-
kenaf (Hibiscus cannabinus)
-
roselle (Hibiscus sabdariffa)
-
lucerne (Medicago sativa)

Fig. 8. Mature caterpillar of tomato pinworm (Keiferia lycopersicella). Note the more uniform purple colour and the less obvious dark anterior line on the prothoracic shield in this specimen. Photo by Alton N. Sparks, Jr., University of Georgia, Bugwood.org - http://www.forestryimages.org/browse/detail.cfm?imgnum=1327086. Under the Creative Commons Attribution 3.0 United States licence.
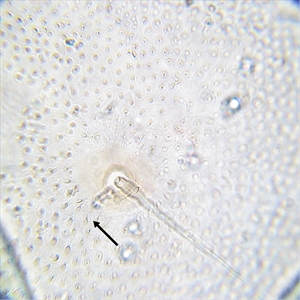
Fig. 9. High magnification view of the posterior abdominal integument of the mature caterpillar of tomato pinworm (Keiferia lycopersicella). The arrow indicates microgranules bearing short microsetae, covering the integument in this area. Photo by permission from Hayden et al. (2013). http://idtools.org/id/leps/micro/factsheet.php?name=%3Cem%3EKeiferia+lycopersicella%3C%2Fem%3E
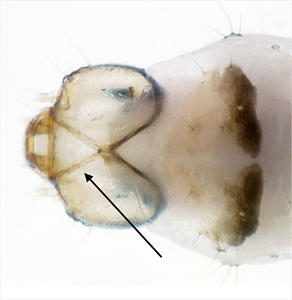
Fig. 10. Dorsal view of head and first thoracic segment of the mature caterpillar of tomato pinworm (Keiferia lycopersicella). Not that the frontoclypeus is longer than wide, and extends all the way to the epicranial notch, a condition typical of prognathous leafminers. Photo by permission from Hayden et al. (2013). http://idtools.org/id/leps/micro/factsheet.php?name=%3Cem%3EKeiferia+lycopersicella%3C%2Fem%3E
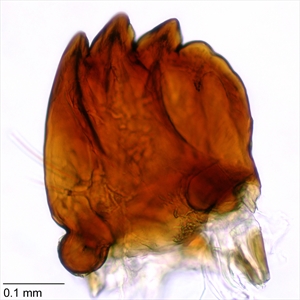
Fig. 12. Mandible of pink bollworm (Pectinophora gossypiella). Photo: T. M. Gilligan & S. C. Passoa, LepIntercept (www.lepintercept.org).
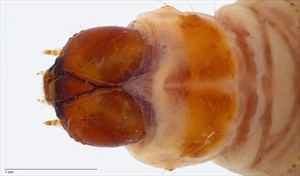
Fig. 14. Head and first thoracic segment of mature larva of pink bollworm (Pectinophora gossypiella). Note the crescent-shaped prothoracic shield typical of this species. Photo: T. M. Gilligan & S. C. Passoa, LepIntercept (www.lepintercept.org).
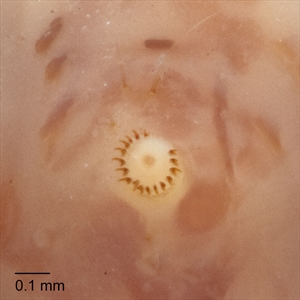
Fig. 15. Ventral view of a ventral proleg of the mature larva of pink bollworm (Pectinophora gossypiella), showing the crochets arranged as a uniordinal penellipse. Photo: T. M. Gilligan & S. C. Passoa, LepIntercept (www.lepintercept.org).
References
Centre for Agriculture and Bioscience International (CABI) (2020a). Invasive Species Compendium. Anarsia lineatella (peach twig borer). https://www.cabi.org/isc/datasheet/5154. Accessed July 2020.
Centre for Agriculture and Bioscience International (CABI) (2020b). Plantwise Knowledge Bank. Anarsia lineatella (peach twig borer). https://www.plantwise.org/knowledgebank/datasheet/25556. Accessed July 2020.
Centre for Agriculture and Bioscience International (CABI) (2020c). Plantwise Knowledge Bank. Keiferia lycopersicella (tomato pinworm). https://www.plantwise.org/knowledgebank/datasheet/5154. Accessed August 2020.
Centre for Agriculture and Bioscience International (CABI) (2020d). Plantwise Knowledge Bank. (pink bollworm) Pectinophora gossypiella. https://www.cabi.org/isc/datasheet/39417 Accessed August 2020.
Gilligan, T. M. & S. C. Passoa. 2014. LepIntercept, An identification resource for intercepted Lepidoptera larvae. Identification Technology Program (ITP), USDA-APHIS-PPQ-S&T, Fort Collins, CO. [accessed at www.lepintercept.org]
Gregersen K., Karsholt O. (2017) Taxonomic confusion around the Peach Twig Borer, Anarsia lineatella Zeller, 1839, with description of a new species (Lepidoptera, Gelechiidae). Nota Lepidopterologica 40(1): 65-85. https://doi.org/10.3897/nl.40.11184
Hayden, J.E., S. Lee, S.C. Passoa, J. Young, J.-F. Landry, V. Nazari, R. Mally, L.A. Somma, & K.M. Ahlmark. 2013. Digital Identification of Microlepidoptera on Solanaceae. USDA-APHIS-PPQ Identification Technology Program (ITP). Fort Collins, CO. [accessed August 2020] http://idtools.org/id/leps/micro/, http://idtools.org/id/leps/micro/factsheet.php?name=%3Cem%3EKeiferia+lycopersicella%3C%2Fem%3E
Heikkilä, M., Mutanen, M., Kekkonen, M. & Kaila, L. (2014) Morphology reinforces proposed molecular phylogenetic affinities: a revised classification for Gelechioidea (Lepidoptera). Cladistics 30: 563–589.
Hodges, R. W. (1999). The Gelechioidea. In: Kristensen, N.P. (Ed.), Handbook of Zoology. 4(35.1). De Gruyter, Berlin, New York.
Huemer P & Karsholt O (2010) Gelechiidae II (Gelechiinae: Gnorimoschemini). In: Huemer P, Karsholt O & Nuss M (Eds) Microlepidoptera of Europe 6: 1–586. [Stenstrup] https://doi.org/10.1163/9789004260986
Karsholt, O., Mutanen, M., Lee, S., & Kaila, L. (2013). A molecular analysis of the Gelechiidae (Lepidoptera, Gelechioidea) with an interpretative grouping of its taxa. Systematic Entomology, 38: 334-348.
MAF Plant Health & Environment Laboratory (2011) Peach Twig Borer (Anarsia lineatella) Updated on 5/7/2014 3:47:33 AM Available online: PaDIL - http://www.padil.gov.au
Passoa, S.C. 1995. Larval and pupal systematics of Nearctic Amphisbatinae and Depressariinae (Lepidoptera; Oecophoridae). Ph.D. Dissertation, University of Illinois, Urbana, pp. 300.
Poe, S. L. (1999) Featured Creatures. Tomato Pinworm. University of Florida. Latest revision: November 2005. Reviewed: June 2020. http://entnemdept.ufl.edu/creatures/veg/tomato/tomato_pinworm.htm
Stehr, F.W., Martinat, P.J., Davis, D.R., Wagner, D.L., Heppner, J.B., Brown, M.E., Toliver, M.E., Miller, J.Y., Downey, J.C., Harvey, D.J., McFarland, N., Neunzig, H.H., Godfrey, G.L., Habeck, D.H., Appleby, J.E., Jeffords, M., Donahue, J.P., Brown, J.W. & Frack, D.C. (1987) Order Lepidoptera, pp 288–596. In Stehr, F. W. (Ed.), Immature Insects. Kendall/Hunt, Dubuque.
Sohn, J., Regier, J. C., Mitter, C., Adamski, D., Landry, J. Heikkilä, M., Park, K., Harrison, T., Mitter, K., Zwick, A., Kawahara, A., Cho, S., Cummings, M. And Schmitz, P. (2016). Phylogeny and feeding trait evolution of the mega-diverse Gelechioidea (Lepidoptera: Obtectomera): new insight from 19 nuclear genes. Systematic Entomology (2016), 41, 112–132 DOI: 10.1111/syen.12143
Trumble, J. (2013) Keiferia lycopersicella. EPPO Bulletin. Volume 43, Issue 1. Accessed August 2020. https://doi.org/10.1111/epp.12026
 Ardozyga stratifera (Gelechiinae)
Ardozyga stratifera (Gelechiinae) Fig. 1. Mature caterpillar of the widespread Australian gelechiid Ardozyga stratifera (Gelechiinae). This species feeds on eucalypts and forms a shelter by tying together leaves along the midline and securing with silk. Photo by Geoff Byrne.
Fig. 1. Mature caterpillar of the widespread Australian gelechiid Ardozyga stratifera (Gelechiinae). This species feeds on eucalypts and forms a shelter by tying together leaves along the midline and securing with silk. Photo by Geoff Byrne. Fig. 2. Mature caterpillar of Anarsia lineatella (peach twig borer) (Gelechiidae: Anacampsinae) in the shoot of an almond tree (Prunus dulcis) in Valtorres, Spain, May 2019. Photo by Carlos Lozano Tomás. Centro de Sanidad y Certificación Vegetal. Gobierno de Aragón (ES).
Fig. 2. Mature caterpillar of Anarsia lineatella (peach twig borer) (Gelechiidae: Anacampsinae) in the shoot of an almond tree (Prunus dulcis) in Valtorres, Spain, May 2019. Photo by Carlos Lozano Tomás. Centro de Sanidad y Certificación Vegetal. Gobierno de Aragón (ES). Fig. 3. Head and prothorax of mature caterpillar of Anarsia lineatella (peach twig borer) (Gelechiidae: Anacampsinae). Note, that the prothoracic spiracle shares the same pinaculum with the three prespiracular lateral setae (indicated with arrow). Photo by Caroline Harding MAF (MAF 2011), under the Creative Commons Attribution 3.0 Australia Licence.
Fig. 3. Head and prothorax of mature caterpillar of Anarsia lineatella (peach twig borer) (Gelechiidae: Anacampsinae). Note, that the prothoracic spiracle shares the same pinaculum with the three prespiracular lateral setae (indicated with arrow). Photo by Caroline Harding MAF (MAF 2011), under the Creative Commons Attribution 3.0 Australia Licence.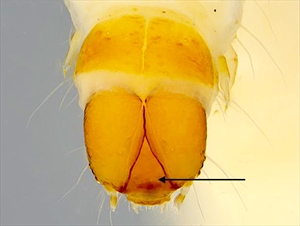 Fig. 4. Dorsal view of the head and prothorax of the mature caterpillar of Anarsia lineatella (peach twig borer) (Gelechiidae: Anacampsinae). Note that the frontoclypeus is longer than wide, and extends over one half the distance to the epicranial notch (indicated by arrow), which are typical gelechiid characteristics. Photo by Caroline Harding MAF (MAF 2011), under the Creative Commons Attribution 3.0 Australia Licence.
Fig. 4. Dorsal view of the head and prothorax of the mature caterpillar of Anarsia lineatella (peach twig borer) (Gelechiidae: Anacampsinae). Note that the frontoclypeus is longer than wide, and extends over one half the distance to the epicranial notch (indicated by arrow), which are typical gelechiid characteristics. Photo by Caroline Harding MAF (MAF 2011), under the Creative Commons Attribution 3.0 Australia Licence.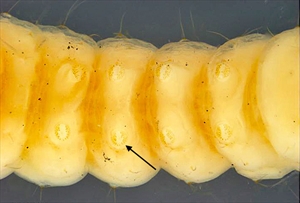 Fig. 5. Ventral view of mature caterpillar of Anarsia lineatella (peach twig borer) (Gelechiidae: Anacampsinae). Note the crochets are arranged in a partially biordinal circle, an arrangement typical of the Gelechiidae (indicated by arrow). Photo by Caroline Harding MAF (MAF 2011), under the Creative Commons Attribution 3.0 Australia Licence.
Fig. 5. Ventral view of mature caterpillar of Anarsia lineatella (peach twig borer) (Gelechiidae: Anacampsinae). Note the crochets are arranged in a partially biordinal circle, an arrangement typical of the Gelechiidae (indicated by arrow). Photo by Caroline Harding MAF (MAF 2011), under the Creative Commons Attribution 3.0 Australia Licence. Fig. 6. Mature caterpillar (preserved) of tomato pinworm (Keiferia lycopersicella). Note the diagnostic, pale prothoracic shield with a black posterior band and purple banding, typical of this species and the prognathous head, characteristic of leaf miners. Photo by permission from Hayden et al. (2013).
Fig. 6. Mature caterpillar (preserved) of tomato pinworm (Keiferia lycopersicella). Note the diagnostic, pale prothoracic shield with a black posterior band and purple banding, typical of this species and the prognathous head, characteristic of leaf miners. Photo by permission from Hayden et al. (2013). Fig. 7. Live mature caterpillar of tomato pinworm (Keiferia lycopersicella). The arrow indicates the diagnostic, pale prothoracic shield with a black posterior band, typical of this species. Note also the typical purple banding on the dorsum. Note the prognathous head, characteristic of leaf miners. Scale = 1 mm. Photo by permission from Hayden et al. (2013).
Fig. 7. Live mature caterpillar of tomato pinworm (Keiferia lycopersicella). The arrow indicates the diagnostic, pale prothoracic shield with a black posterior band, typical of this species. Note also the typical purple banding on the dorsum. Note the prognathous head, characteristic of leaf miners. Scale = 1 mm. Photo by permission from Hayden et al. (2013). Fig. 11. Live mature caterpillar of tomato pinworm (Keiferia lycopersicella) and damage caused by the caterpillar mining and feeding in a tomato leaf. Photo by permission from Hayden et al. (2013).
Fig. 11. Live mature caterpillar of tomato pinworm (Keiferia lycopersicella) and damage caused by the caterpillar mining and feeding in a tomato leaf. Photo by permission from Hayden et al. (2013). Fig. 13. Mature larvae of pink bollworm (Pectinophora gossypiella) emerging from a cotton boll. ©Peggy Greb/USDA Agricultural Research Service/Bugwood.org - CC BY 3.0 US.
Fig. 13. Mature larvae of pink bollworm (Pectinophora gossypiella) emerging from a cotton boll. ©Peggy Greb/USDA Agricultural Research Service/Bugwood.org - CC BY 3.0 US.