Background
The Crambidae is a large, diverse and ubiquitous family of moths that currently comprises 11,500 species globally, with at least half that number again undescribed. The Crambidae and the Pyralidae constitute the superfamily Pyraloidea. Crambid larvae are concealed feeders with a great diversity in feeding habits, shelter building and hosts, such as: leaf rollers, shoot borers, grass borers, leaf webbers, moss feeders, root feeders that shelter in soil tunnels, and solely aquatic life habits. Many species are economically important pests in crops and stored food products.
Subfamilies
Until recently, the Crambidae was treated as a subfamily under the Pyralidae (snout moths or grass moths). Now they form the superfamily Pyraloidea with the Pyralidae. The Crambidae currently consists of the following 14 subfamilies:
- Acentropinae
- Crambinae
- Cybalomiinae
- Glaphyriinae
- Heliothelinae
- Lathrotelinae
- Linostinae
- Midilinae
- Musotiminae
- Odontiinae
- Pyraustinae
- Schoenobiinae
- Scopariinae
- Spilomelinae
Short Description
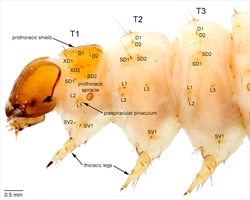
Crambid caterpillars are generally cylindrical, with a semiprognathous head and only primary setae (Fig 1). They are often plainly coloured (Fig. 16, Fig. 19), but can be patterned with longitudinal stripes and pinacula that may give them a spotted appearance (Fig. 10, Fig. 11, Fig. 14, Fig. 22). Prolegs may be reduced in borers (Fig. 16). More detailed descriptions are provided below.
This factsheet presents, firstly, diagnostic features for the Pyraloidea (Pyralidae and Crambidae) and then the Crambidae. Information and diagnostic features are then provided for crambids listed as priority biosecurity threats for northern Australia.
Diagnosis
Diagnosis of the Pyraloidea – Pyralidae and Crambidae
Adapted from Stehr et al. (1987) and Solis (1999).
Almost all pyraloid larvae are concealed feeders e.g. leaf rollers, leaf webbers, leaf miners, borers, root feeders, seed and fruit feeders, within bird nests, or under silk coverings.
Pyraloid larvae can be distinguished from other Lepidoptera on the following combination of characters:
- Two L setae on the prespiracular plate of T1 (Fig. 2). Pyraloids (pyralids and crambids), noctuids, and other macrolepidopteran (Macroheterocera) groups have two L setae on the prespiracular plate of TI; whereas many microlepidopteran groups have three L setae in the same location. The Pyraloidea plus the Macroheterocera are monophyletic and are known collectively as eared moths.
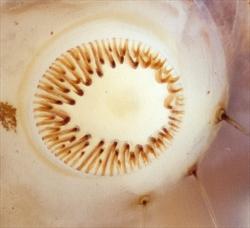
- Crochets in a complete circle or penellipse (Fig. 3, Fig. 4). Pyraloids can be distinguished from noctuoids (moths belonging to the superfamily Noctuoidea) on this character because noctuoids usually have crochets arranged in a mesoseries, whereas crochets in pyraloids are arranged in a complete circle or penellipse.
- Three subventral (SV) setae on abdominal segments A3 to A6. Larvae of the Carposinidae also have two L setae on the prespiracular plate of T1 and crochets arranged in a circle. They can be distinguished from pyraloids because they usually have four or more subventral setae, but this may vary from segment to segment.
Crambidae
Adapted from Stehr et al. (1987) and Solis (1999).
Short Description
Crambid caterpillars are generally cylindrical and tapered anteriorly and posteriorly (Fig. 1). They have the usual number of prolegs (Fig. 1), which can sometimes be reduced but still retain crochets. Crochets are usually arranged in a circle or modified circle and in the mature larvae, multiordinal (Fig. 3, Fig. 4). Well-developed primary setae are present, often from distinct pinacula (Fig. 1, Fig. 2). There are two lateral setae on the prespiracular plate of T1 (Fig. 2).
Diagnosis
- Absence of sclerotised ring around SD1 on A8 in the Crambidae (Fig. 5). The presence of this character distinguishes the Pyralidae (Fig. 6) from the Crambidae, although it is also usually missing from the Phycitinae (Pyralidae). The phycitine, carob moth Ectomyelois ceratoniae, illustrated in (Fig. 6), is an exception. Note that the ring is sometimes difficult to detect and is sometimes a shiny, white, unsclerotised, translucent ring (e.g. greater wax moth, Galleria mellonella (Pyralidae: Galleriinae) (Solis 2007)).
- Presence of one or two L seta(e) on A9 in the Crambidae (Fig. 5) This character distinguishes the Crambidae from the Pyralidae, in which there are three (or occasionally two*) L setae on A9.
*For example, there are two A9 L setae in the pyralid Etiella zinckenella (pulse pod borer moth) (Phycitinae), which has been introduced into Australia (see also Fig. 6).
Detailed Description
Adapted from Stehr et al. (1987) and Solis (1999).
Body - general description: Cylindrical, tapered anteriorly (Fig. 1) and posteriorly with usually well-developed prolegs on the A3-A6 and A10 (Fig. 1), sometimes bearing tracheal gills (Acentropinae: Nymphulini), or a bag-like structure between the thoracic legs (Schoenobiinae). The prolegs are usually well developed on segments A3-A6 and A10 (Fig. 1). The epidermis of the body is smooth to slightly granular and usually more or less unicolourous, with the venter frequently paler. Some species have longitudinal stripes and some have contrasting dark pinacula (Fig. 10, Fig. 11, Fig. 14). Tonofibrillary platelets are associated with the epidermis and may be a distinctive pigmented feature on the head, prothoracic and anal shields of many pyraloids. The usual complement of primary setae is present in most species but reduced in a few. The spiracles are usually elliptical (Fig. 1), with those on T1 and A8 frequently larger.
Head: More or less evenly rounded, and semiprognathous (Fig. 1, Fig. 2, Fig. 7) in most species but prognathous and flattened in leaf miners (in a few Odontiinae and some Nymphulini (in Acentropinae)). There are six stemmata. The mandibles are usually simple. The maxillae are usually simple with sensilla trichodea.
Thorax: Usually has a distinct prothoracic shield, characteristically with six setae on each side (Fig. 2). Almost all species have two L setae on the prespiracular plate of T1 (Fig. 2, Fig. 7) (some Nymphulini (in Acentropinae)) have a very small L seta that is easily overlooked). D1 and D2 are present and usually adjacent to each other on T2 and also on T3 (Fig. 2, Fig. 7). SD1 and SD2 are present and adjacent to each other on T2 and also on T3 (Fig. 2). The L group is usually trisetose on T2 and T3 (Fig. 2) (but apparently bisetose in some Nymphulini (in Acentropinae) and Schoenobiinae). The SV group is usually bisetose on T1 (Fig. 2), and unisetose or bisetose on T2 and T3 (Fig. 2, Fig. 7). A few (some Nymphulini (in Acentropinae)) have simple or compound gills on T2 and T3, and the schoenobiines have anteromesial sacs or gibbosities associated with the thoracic legs.
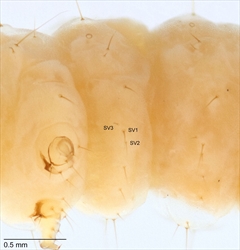
Abdomen: D1 and D2 usually on all segments, with D1 more dorsad and anteriad than D2, except on A9 where D1 is usually ventrad of D2 (D1 or D2 are very small or missing on the abdominal segments of some Nymphulini (in Acentropinae) and Schoenobiinae). SD1 is present on all segments (Fig. 5, Fig. 6) (but is small in some Nymphulinae). SD2 is on all segments except A9 and A10, but is very small. On A1-A8, L1 and L2 are usually approximate and more or less ventrad of the spiracle (in some Nymphulini (in Acentropinae) and possibly some Schoenobiinae, L1 (or L2) is strongly reduced or absent). Only one or two L setae are present on A9 (Fig. 5, Fig. 6). On A1-A8, L3 is below L1 and L2. SV is bisetose or trisetose on A1-A6 (Fig. 8). SV is unisetose to trisetose on A7-A9. V1 is present on all segments (Fig. 8).
Crochets are uni- to triordinal on the prolegs on abdominal segments A3-A6 (Fig. 3Fig. 4). The crochets are most often arranged in the form of circles or penellipses (Fig. 3, Fig. 4); in the Nymphulini (Acentropinae) they can be incomplete ellipses that are open laterally and mesally. A10 usually has a relatively indistinct suranal plate (Fig. 5, Fig. 6), usually bearing eight setae. The crochets on the proleg on A10 are usually bi- to triordinal. Typically, nine setae are on and near the anal proleg. Some nymphulines (Acentropinae) have simple or compound gills on some or all of the abdominal segments.
Crambidae Subfamilies
For a key to Crambidae subfamilies, see Solis (2006), https://www.ars.usda.gov/ARSUserFiles/12754100/PyraloideaKey.pdf
Diagnosis of selected subfamilies
Adapted from Solis (2006)
- Acentropinae
- With lateral gills on body.
- Schoenobiinae
- Crambinae
- Pyraustinae, Glaphyriinae, Glaphyriinae
- A pair of transverse plates is present that is posterior to the dorsal pinaculum on T2 (second thoracic segment), or, these plates are completely absent. Crochets are usually arranged in a mesal penellipse.
Species of Biosecurity Concern
THE FOLLOWING SPECIES ARE OF BIOSECURITY CONCERN TO NORTHERN AUSTRALIA
The following seven species of Crambidae are considered to be of biosecurity concern for northern Australia. Five belong to the very large genus Chilo (stemborers) (Crambinae).
Subfamily Crambinae
Crambinae are of two general habits: root-feeders or ground-living leaf feeders, on grasses or mosses, rarely on other plants; and stem-borers in grasses, sedges, and rushes. Many species are of economic importance.
Diagnosis of the Crambinae
- A single transverse plate is present that is posterior to the dorsal pinaculum on T2 (second thoracic segment).
- Crochets in a complete circle (Fig. 3).
Chilo (stemborers)
Description
Caterpillars of Chilo species are stemborers that attack grasses (Gramineae, Poaceae), such as corn, sugarcane, rice, sorghum, millet and other important crops. This association has facilitated accidental introductions of many of these pest species into other geographical areas. Chilo contains 41 species, which are mainly distributed in the Ethiopian and Oriental Regions.
Mature Chilo caterpillars are cylindrical, with dark- to reddish-brown head capsules. The body is usually pale, often with dark longitudinal stripes and conspicuous pinacula with gives a spotted appearance. The prothoracic shield is dark and reasonably well-developed. Crochets are arranged in a circle and the prolegs are likely to be under-developed (Fig. 10, Fig. 11, Fig. 14, Fig. 16).
Approximately 12 species of this genus are associated with sugarcane, with the five listed here considered to be priority pests of biosecurity concern for northern Australia.
Diagnosis
Adapted from Meijerman & Ulenberg (1996).
These characters distinguish Chilo larvae from the following common African stemborer larva: Coniesta (Crambidae), Scirpophaga (Crambidae), Eldana (African sugar-cane borer) (Pyralidae), Maliarpha (Pyralidae), Sesamia (Erebidae) and Busseola (Erebidae). The characters may not necessarily distinguish Chilo from other taxa but are still useful in identification.
- Crochets
- bi- or triordinal, rarely uniordinal.
- Thorax chaetotaxy
- T1 (first thoracic segment)
- T2 (second thoracic segment) and T3 (third thoracic segment)
- with a large, dark, dorsal, asetose tubercle (with seta absent).
- with 2 SV setae.
- T3 (third thoracic segment)
- With one dorsal microscopic seta.
- Abdomen
- A1-A7 with only 1, or no, lateral asetose tubercle (with setae absent).
- A1-A8 with D2 shorter than D1.
- A9 with SD1 smaller than D1.
***
Chilo auricilius (sugarcane internode borer, gold-fringed rice stemborer or terai borer)
These caterpillars are stem borers, which tunnel into and feed on the stems of plants of several grass families. Host plants include sugarcane, rice and maize.
Description
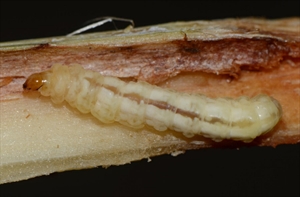
Mature larvae are around 25-30 mm in length, creamy-white with five violet longitudinal stripes. The head is brown. Crochets multiordinal, in complete circles. Spiracles have grey rims (peritremes).
Biology and Feeding Damage
Sugarcane: Damage is similar to that caused by other stem borers, i.e.:
- death of central tissue
- flowerhead fails to develop
- emerging panicles are empty and white.
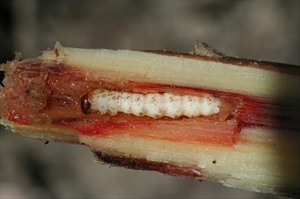
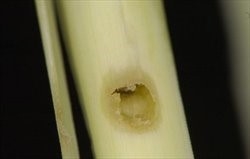
On young plants, larvae kill the leaves and shoots, sometimes producing ‘dead hearts’. In older cane there may be no noticeable symptoms until the leaf sheaths are stripped off, when the gallery entrances become apparent (Fig. 9). Damage becomes more visible as dead tops of stalks and bored stems.
For a comprehensive list of symptoms and other information, see: https://www.cabi.org/isc/datasheet/18759
Current Distribution
Asia and Oceania
- India
- Taiwan
- Bhutan
- Sri Lanka
- Sulawesi
- Borneo
- Sangir Island
- The Moluccas
- Bangaldesh
- Cambodia
- China
- Indonesia
- Laos
- Malaysia
- Nepal
- Pakistan
- Philippines
- Thailand
- Vietnam
- Papua New Guinea
Caterpillar host plants
- rice (Oryza sativa)
- sugarcane (Saccharum officinarum)
- sorghum (Sorghum bicolor)
- maize (Zea mays)
***
Chilo infuscatellus (shoot borer, yellow top borer, sugarcane stem borer)
Caterpillars of this species are key pests of sugarcane, but they also sometimes feed on other grasses. This species is a major pest of sugarcane in India.
Description
The mature larvae are greyish-white with five violet longitudinal stripes. The head and prothoracic shield are dark brown. The body is conspicuously spotted from moderately large brown pinacula (Fig. 10) but is also similar in this regard to C. partellus (see Fig. 11) and C. sacchariphagus (see Fig. 14).
Biology and Feeding Damage
Damage is similar to that caused by other stem borers, i.e.:
- external feeding on leaves
- internal feeding on and in leaves
- internal feeding in stems
When young, caterpillars eat small holes in leaves and in particular the sheaths. When older and larger, they attack the tips of shoots, often killing them, and then moving on to the stems. In the stems they form galleries, which become apparent from frass dropping from the entrance holes. Brittle stems and dead hearts are characteristic of damage.
Caterpillar host plants
Poaceae (grasses):
- oats (Avena sativa)
- citronella grass (Cymbopogon winterianus)
- Bermuda grass (Cynodon dactylon)
- Java grass (Cyperus rotundus)
- jungle rice (Echinochloa colona)
- barley (Hordeum vulgare)
- rice (Oryza sativa)
- millet (Panicum)
- pearl millet (Pennisetum glaucum)
- sugarcane (Saccharum officinarum)
- sorghum (Sorghum bicolor)
- maize (Zea mays)
***
Chilo partellus (spotted stalk borer)
This species, which is indigenous to Asia, is a key pest of maize, sorghum and rice, and sometimes sugarcane.
Description
Adapted from Meijerman & Ulenberg (1996).
Diagnosis
- Body (general)
- brown pinacula – from which is derived its common name, spotted stalk borer (Fig. 11) (but similar to Chilo infuscatellus, see Fig. 10, and C. sacchariphagus, see Fig. 14).
- Thorax
- T2 (second thoracic segment) and T3 (third thoracic segment) with subventral asetose tubercles (with setae absent)
- Abdomen
- A1-A7 – with lateral asetose tubercles (without setae).
- A9 – D1, D2 and SD setae usually together on one large pinaculum.
Mature larvae are around 25-30 mm in length, creamy-white with five violet longitudinal stripes. The head is brown. Crochets multiordinal, in complete circles. Spiracles have grey rims (peritremes).
General Description
The body of the caterpillar is cream-coloured to yellowish-brown apart from four, quite faint, dorsal, reddish-brown or purple longitudinal stripes and is conspicuously spotted from reasonably large brown pinacula (Fig. 11) (similar to Chilo infuscatellus, Fig. 10, and C. sacchariphagus, Fig. 14). C. partellus resembles C. infuscatellus except that the dorsal stripes are more prominent in C. infuscatellus (Fig. 10). Diapausing larvae can be pale with or without stripes. The head, prothoracic shield and suranal plate are brown. The body has a large number of asetose tubercles.
Biology and Feeding Damage
Damage is similar to that caused by other stem borers, i.e.:
- external feeding on leaves (Fig. 12)
- internal feeding on and in leaves
- internal feeding on and in stems and maize cobs (Fig. 13)
Leaves are usually destroyed by feeding and the plant killed by the destruction of growing points (dead hearts). The first symptoms are irregularly shaped pinholes or ‘shot’ holes in lines and/or patched of transparent leaf epidermis (‘windows’) caused by feeding in the whorl. These later become elongated lesions on the leaves (Fig. 12). Infested plants deteriorate and appear ragged.
Older larvae bore into stems (Fig. 13). Damage to flowers may interfere with grain formation causing damage such as ‘chaffy heads’ in sorghum.
(CABI 2019c).
Current Distribution
Afrotropical Region
- Comoro Is.
- Ethiopia
- Kenya
- Lesotho
- Madagascar
- Malawi
- Mayotte
- South Africa
- Sudan
- Tanzania
- Uganda
Asia
- India
- Pakistan
Caterpillar host plants
- rice (Oryza sativa)
- sugarcane (Saccharum officinarum)
- sorghum (Sorghum bicolor)
- maize (Zea mays)
- finger millet (Eleusine coracana)
- thatch grass (Hyparrhenia rufa)
- Guinea grass, green panic grass (Megathyrsus maximus)
- Napier grass, elephant grass or Uganda grass (Pennisetum purpureum)
- pearl millet (Pennisetum glaucum)
- foxtail millet (Setaria italica)
- Hemarthria sibirica
- Vossia cuspidata
***
Chilo sacchariphagus (spotted borer)
Caterpillars of this species are major pests of sugarcane, but sometimes feed on other grasses. There are three recognised subspecies: C. sacchariphagus sacchariphagus, C. sacchariphagus indicus and C. sacchariphagus stramineellus.
Description
Mature caterpillars are cream-coloured with four brown longitudinal stripes. This species has very conspicuous dark brown pinacula typical of the genus and also found in varying degrees of prominence in C. infuscatellus (Fig. 10) and C. partellus (Fig. 11) and C. terrenellus (Fig. 16). This strongly marked caterpillar can be distinguished from the other Chilo species here on its larger and darker dorsal pinacula and the relatively broad longitudinal stripes. The head capsule and prothoracic shield are brown (Fig. 14).
Biology and Feeding Damage
Damage is similar to that caused by other stem borers, i.e.:
- external feeding on leaves
- internal feeding on and in leaves
- necrotic areas in leaves
- internal feeding in stems (Fig. 15)
- damage to growing point - dead heart, rot
- whole plant dead heart
Feeding by larvae cause leaves to unfold and repetitive patterns of holes. Feeding on growing points causes the formation of ‘dead hearts’. Tunnelling in internodes and stems produces galleries. Frass can be seen falling from the entrance holes. The target area of larvae is usually the internodes at the top of canes. Usually only two larvae per internode are found feeding together. Damage is most apparent during dry periods.
(CABI 2019d)
Current Distribution
- Malaysia
- Indonesia
- India
- China
- Taiwan
- Bangladesh
- Brunei Darussalam
- Cambodia
- Indonesia
- Java
- Mauritius
- Réunion Island
- Madagascar
- Comores
- Mafambisse
- Mozambique
- Marromeu
- South Africa
- Tanzania
- Iran
- Japan
- Laos
- Malaysia
- Pakistan
- Philippines
- Singapore
- Sri Lanka
- Thailand
- Vietnam
(see CABI 2019d)
Caterpillar host plants
- rice (Oryza sativa)
- sugarcane (Saccharum officinarum)
- sorghum (Sorghum bicolor)
- maize (Zea mays)
***
Chilo terrenellus (stem borer, sugarcane borer)
This species is apparently confined to sugarcane of which it is a key pest.
Description
Mature caterpillars are white, with a dark reddish-brown head capsule and brown prothoracic shield, and are 20-30 mm long (Fig. 16) (Jackson 2017). This species has the brown pinacula typical of the genus and also found in C. infuscatellus (Fig. 10), C. partellus (Fig. 11) and C. sacchariphagus (Fig. 14), but lacks the longitudinal stripes of other species (Carmichael et al. 2005), and is overall less strongly marked than the other species described here.
Biology and Feeding Damage
Immature larvae are gregarious, feeding together on leaves. As they mature, caterpillars feed singly and bore into stems (Fig. 16, Fig. 17) or leaf sheaths (Jackson 2017). Prolegs are reduced. The caterpillars normally do not leave frass inside the stalks like other cane borers (e.g. Sesamia (Noctuidae)).
For more information see: http://www.pestnet.org/fact_sheets/sugarcane_borer_277.htm
Feeding Damage
Damage is similar to that caused by other stem borers, i.e. internal feeding in stems.
Caterpillars of this species tunnel into the stalks of sugarcane (Fig. 16, Fig. 17), resulting in dead tops and broken stalks, ‘dead hearts’ and reduced sugar content (Jackson 2017). This may also cause invasion and damage by other pests and pathogens.
Current Distribution
- Papua New Guinea
- Torres Strait Islands
Caterpillar host plants
- sugarcane (Saccharum officinarum)
- robust cane (Saccharum robustum)
- lowland pitpit (Saccharum edule)
*****
Subfamily Schoenobiinae
Scirpophaga excerptalis, sugarcane top borer belongs to the tribe Schoenobiinae, and is a serious pest of sugarcane in many countries. There are 21 species in this tribe in Australia.
Diagnosis of the Schoenobiinae
- With a membranous sac or gibbosity anterior to the prothoracic (T1) coxae.
Scirpophaga excerptalis (sugarcane top borer, white top borer)
Diagnosis
Adapted from Meijerman & Ulenberg (1996).
The following characteristics may differentiate Scirpophaga excerptalis from Chilo and other stemborer larvae that attack sugarcane:
- Thorax
- T1 (prothorax) - Lateral pinaculum anteroventral to the spiracle.
- T2 (second thoracic segment) and T3 (third thoracic segment)
- with no large, dark, asetose, dorsal tubercles (with setae absent)
- two lateral (L) setae
- T1 (prothorax) - with a membranous sac or gibbosity anteriorto the prothoracic coxae
- Abdomen
- A1-A8
- with D1 shorter than D2
- one macroscopic subdorsal seta (SD1) and one microscopic subdorsal seta.
- SD2 ventral to SD1
- two lateral (L) setae.
- A7 with three subventral (SV) setae
- A9
- with D1 shorter than seta SD1
- one lateral (L) setae
- A1-A8
Biology and Feeding Damage
Damage is similar to that caused by other stem borers, i.e.:
- internal feeding; boring (Fig. 18)
- Leaves - external and internal feeding (Fig. 19)
- dead heart in stems and growing points (Fig. 20)
- Young caterpillars eat through young, rolled leaves, causing ‘shothole’ damage (Fig. 19). They then penetrate into the plant heart by eating along the midrib of the leaf, causing ‘deadheart’ (Fig. 20)
(CABI 2019e).
Current Distribution
- Angola
- Gambia
- Ghana
- Ivory Coast
- Kenya
- Madagascar
- Malawi
- Mauritius
- Nigeria
- Reunion
- Senegal
- Tanzania
- Uganda
- Zambia
- Zanzibar
- China
- Taiwan
- Japan
- Pakistan
- India
- Nepal
- Bangladesh
- Thailand
- Vietnam
- Singapore
- western Malaysia
- Java
- Sumba Island
- Timor
- Buru
- Adonara Island
- Ambon Island
- The Philippines
- New Guinea
- New Hannover
- New Britain
- New Ireland
- Australia
- Solomon Islands
Caterpillar host plants
- rice (Oryz sativa)
- robust cane (Saccharum robustum)
- sugarcane (Saccharum officinarum)
- wheat (Triticum)
*****
Subfamily Odontiinae
Deanolis sublimbalis (red banded mango caterpillar)
Description
The mature caterpillar has 11 transverse, broad, crimson bands with pale pink bands in between. The head capsule is golden brown. Length is approximately 20 mm (Fig. 21, Fig. 22) (Walker et al. 2005).
Biology and Feeding Damage
Young caterpillars feed on the fruit pulp and form a network of tunnels, which may cause the fruit to collapse. Mature caterpillars feed on seed (Fig. 22). Black stains on mangos are a sign of infestation.
Current Distribution
- India
- Indonesia
- Papua New Guinea
- Burma
- Thailand
- Chin
- Brunei
- the Philippines
- Australia – Cape York Peninsula.
Caterpillar host plants
Sugarcane
- sugarcane (Saccharum officinarum)
- mango (Mangifera indica)
- Mangifera minor
- kwini (Mangifera odorata)
- purple nut sedge (Cyperus rotundus)
References
Anderson, S. & Tran-Nguyen, L. (2012a) Gold-fringed Rice Borer (Chilo auricilius) .Updated on 2/24/2012 7:23:07 PM Available online: PaDIL - http://www.padil.gov.au.Accessed June 2019.
Anderson, S. & L. Tran-Nguyen (2012b) Spotted Borer (Chilo sacchariphagus) Updated on 2/27/2012 10:32:21 AM Available online: PaDIL - http://www.padil.gov.au.
Anderson, S. & L. Tran-Nguyen (2012c) Top Shoot Borer (Scirpophaga excerptalis) Updated on 2/24/2012 7:21:31 PM Available online: PaDIL - http://www.padil.gov.au.
Carmichael, A., Anderson, S., & Tran Nguyen, L. (2005) Stem borer (Chilo terrenellus). Updated on 2/27/2012 10:34:21 AM Available online: PaDIL - http://www.padil.gov.au.
Centre for Agriculture and Bioscience International (CABI) (2019a). Invasive Species Compendium. Chilo auricilius (gold-fringed rice borer) https://www.cabi.org/isc/datasheet/18759. Accessed June 2019.
Centre for Agriculture and Bioscience International (CABI) (2019b). Plantwise Knowledge Bank. Chilo infuscatellus (yellow top borer) https://www.plantwise.org/knowledgebank/datasheet/18767. Accessed June 2019.
Centre for Agriculture and Bioscience International (CABI) (2019c). Plantwise Knowledge Bank. Chilo partellus (spotted stem borer) https://www.plantwise.org/knowledgebank/datasheet/12859. Accessed June 2019.
Centre for Agriculture and Bioscience International (CABI) (2019d). Invasive Species Compendium. Chilo sacchariphagus (spotted borer) https://www.cabi.org/isc/datasheet/44558. Accessed June 2019.
Centre for Agriculture and Bioscience International (CABI) (2019e). Invasive Species Compendium. Scirpophaga excerptalis (white top borer) https://www.plantwise.org/knowledgebank/datasheet/49051. Accessed June 2019.
Gilligan, T. M. & S. C. Passoa (2014). LepIntercept, An identification resource for intercepted Lepidoptera larvae. Identification Technology Program (ITP), USDA/APHIS/PPQ/S&T, Fort Collins, CO. Accessed at idtools.org.
Jackson, G. (2017). Pacific Pests and Pathogens - Fact Sheets. Sugarcane borer (277). http://www.pestnet.org/fact_sheets/sugarcane_borer_277.htm. Accessed June 2019.
Medson C., Peterson, G., (2019). Breeding and Agronomy, in Sorghum and Millets (Second Edition). AACC International.
Meijerman, L. and Ulenberg, S. A. (1996). Identification of African stemborer larvae (Lepidoptera: Noctuidae, Pyralidae) based on morphology. Bulletin of Entomological Research. 86: 567-578.
Plant Health Australia. Cane borers. http://www.planthealthaustralia.com.au/pests/cane-borers/ Accessed June 2019.
Prasad, G.S. Babu, K.S. (2016) Insect pest resistance in pearl millet & small millets, in Biotic Stress Resistance in Millets, Academic Press.
Sallam, M. S. & Allsopp, P. G. (2002) BSS249 Preparedness for Borer Incursion Chilo Incursion Management Plan Version 1. Bureau of Sugar Experiment Stations, Publication Project Report PR02008. Queensland, Australia
Solis, M.A. (1999) Key to selected Pyraloidea (Lepidoptera) larvae intercepted at U.S. Ports of entry: Revision of Pyraloidea in “Keys to some frequently intercepted lepidopterous larvae” by D. M. Weisman, 1986. Proceedings of the Entomological Society of Washington, 101(3), 645–686.
Solis, M.A. (2006). Key to selected Pyraloidea (Lepidoptera) larvae intercepted at U. S. Ports of entry: Revision of Pyraloidea in “Keys to some frequently intercepted lepidopterous larvae” by Weisman 1986 (updated 2006) .
Solis, M.A. (2007) Phylogenetic studies and modern classification of the Pyraloidea (Lepidoptera). Revista Colombiana de Entomología 33(1): 1-9
Stehr, F.W., Martinat, P.J., Davis, D.R., Wagner, D.L., Heppner, J.B., Brown, M.E., Toliver, M.E., Miller, J.Y., Downey, J.C., Harvey, D.J., McFarland, N., Neunzig, H.H., Godfrey, G.L., Habeck, D.H., Appleby, J.E., Jeffords, M., Donahue, J.P., Brown, J.W. & Frack, D.C. (1987) Order Lepidoptera, pp 288–596. In Stehr, F. W. (Ed.), Immature Insects. Kendall/Hunt, Dubuque.
Walker, K., S. Anderson & L. Tran-Nguyen (2005) Red banded mango caterpillar (Deanolis sublimbalis) Updated on 2/24/2012 7:22:31 PM Available online: PaDIL - http://www.padil.gov.au