|
| Kingdom |
Fungi |
| Phylum |
‘mitosporic fungi’ |
| Class |
Coelomycetes |
| Order |
Sphaeropsidales |
| Family |
Sphaeropsidaceae |
Java black rot is one of the most significant postharvest diseases of
sweetpotato. It can damage a huge quantity of storage roots depending on
the length of time in which they are in storage. The disease can also be severe
in seedbeds when infected roots are used as a source of planting material.
Although there is no economic estimate of damage, it is evident that under
certain environmental and storage conditions significant yield and postharvest
losses are possible.
The disease is present worldwide.
Lasiodiplodia theobromae is a fungus that grows well in a culture medium
(potato-dextrose-agar) in the laboratory, forming fluffy gray colonies initially
which become black with age. The mycelium
is grey to black. A few days after infection the mycelium forms black stromatic
structures containing pycnidia. The pycnidia
are round or elongated, ostiolated, generally aggregated, usually setose, up to
5 mm in size. The conidiophores are
simple, rarely branched, hyaline,
cylindrical, forming a mat in the inner surface of pycnidia. The conidia
which arise from the tip of conidiophores are hyaline, unicellular, and
granulated when young, becoming cinnamon to fawn, bicellular with longitudinal
striae when mature and measure 20-30 x 10-15 µm. They are somewhat subovoid to
ellipsoid-oblong in shape.
The most obvious symptoms are those found in fleshy roots few days after
harvest. Brown to reddish brown, round sunken lesions with solid black centre,
surrounded by soft, pinkish ring of decaying tissues are observed. Soon after,
the lesions become hard, sunken and completely blackened due to the presence of
mature mycelium and stromatic tissue. When infection starts in one or both ends
of the fleshy root, the entire root dries out and mummifies. During the drying
process, black dome-shaped structures bearing pycnidia emerge through root
periderm and an abundance of black powdery spores is shed.
In early stages of infection the symptoms of Java black rot can be confused
with those caused by Ceratocystis fimbriata (black rot) and
Macrophomina phaseolina (charcoal rot). However, lesions caused black
rot are usually confined to the outer layers, in distinct circular lesions.
Charcoal rot initially spreads through the outer cambial layer without forming
superficial lesions. Java black rot spreads usually from one end of the
root, through all layers of tissue. In some cases, the rot is limited to one end
of the root and does not spread further.
Java black rot occurs in seedbeds, attacking underground parts of the plant
through soil borne conidia when infected sprouts have been used as propagation
material.
Fleshy roots are usually infected in the field through wounds made during
harvest by inoculum present in the soil or from the infected mother plant.
Secondary infections occur in storage when insects carrying the
spores infest the storage roots.
L. theobromae requires warm temperatures (20-30oC). Humidity
is not crucial, however, when it is too high the disease does not develop.
When infected fleshy roots are stored after harvest, the fungus starts
developing and after one week or two. A black pimple-like growth is observed on
the surface of the roots. These are pycnidia, containing thousands of conidia,
which are the propagation structures of the fungus.
Due to its ability to live on decaying matter L. theobromae survives
in the soil on plant refuse for several years.
Cacao (die-back), citrus (stem end rot of the fruit), banana (finger rot),
avocado (stem end rot), mango (stem end rot), onion (collar rot), apple (stem
rot), cacao (pod rot), yam, cassava, groundnut and melon.
Cultural control
Use of healthy roots or cuttings as propagation material.
Use of transplants cut above the soil line.
Avoid wounding the roots during harvest and handling.
Curing roots before storage. Storage roots should be cured at 30-34oC
and 90% relative humidity for 4-7 days depending on the cultivar. After curing,
maintain roots at 15-16oC during storage.
Use of clean and disinfected storage containers.
Crop rotation for 3-4 years, if possible.
Host- plant resistance
Differences in susceptibility have been noted among lines and cultivars from
studies done in different countries.
Chemical control
Dipping propagation material (fleshy roots, sprouts) in Benomyl or Captan. To
prevent rotting, the same fungicides are used as a spray for roots before
bedding or storing.
Ames, T., N.E.J.M. Smit, A.R. Braun, J.N. O’Sullivan and L.G. Skoglund.
1996. Sweetpotato: Major pests, diseases, and nutritional disorders.
International Potato Center (CIP). Lima, Peru. 152 p.
Clark, C.A. and J.W. Moyer. 1988. Compendium of sweetpotato diseases. 1988.
APS Press. 74 p.
Holliday, P. 1980. Fungus diseases of tropical crops. Cambridge University
Press. 607 p.
Palomar, M.K., A.D. Solis and H.S. Bandala. 1980. Sweetpotato storage root
rot disease in the Philippines. Ann. Trop. Res. 2:111-121.
Punithalingam, E. 1976. Botryodiplodia theobromae. CMI Descriptions of
Pathogenic Fungi and Bacteria No. 519.
Vasquez , E.A. and
C.E. Sajise. 1989. Pests of sweetpotato: Insects, mites and diseases. PRIS and
PRCRTC, Visayas State College of Agriculture, Baybay, Leyte, Philippines. 66 p.
Contributed
by: Teresa Ames |
Taxonomy
Economic
importance
Geographical
distribution
Morphology
Symptoms
Biology
and ecology
Host
range
Management
References

Internal
and external views of storage roots with Java black rot (J. Lo,
APS).
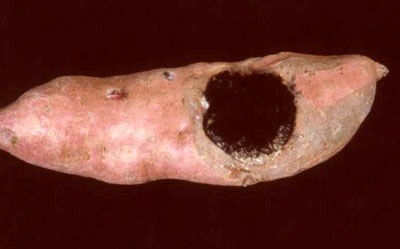
Black
sunken lesion on storage root (T. Ames).

Lesion restricted to one end of a storage root (C. A. Clark).

Black stromatic masses erupt through the root surface, and shed black
powdery spores (C. Clark, APS). |

